Research Highlights
Trk-ing down castration-resistant prostate cancer: 3D organoid models reveal a new drug target for an aggressive form of prostate cancer.
Three-dimensional cancer-derived organoids introduce TrkA as a promising new biomarker and target for a nearly incurable disease.
Institution:
University of Campania
Team:
Marzia Di Donato, Gustavo Cernera, Antimo Migliaccio*and Gabriella Castoria *
Application:
3D tissue model to evaluate role of a gene in tumor progression
Disease model:
Prostate cancer
Cell types:
Human PC-derived DU145 cells
Hydrogel:
VitroGel® RGD (TWG003)
Prostate cancer is one of the most prevalent types of cancer, and the leading cause of death in men living “Western” lifestyles. Prostate cancer is slow-growing and asymptomatic, and therefore, difficult to detect. Once detected, patients undergo multiple combination treatments including prostatectomy and chemotherapeutic treatment. While these treatments can moderate tumor growth, prostate cancer is notorious for developing chemotherapeutic resistance. When primary therapies fail, patients often develop an almost incurable, highly aggressive form of prostate cancer known as castration-resistant prostate cancer (CRPC), characterized by testosterone-independent tumor growth, and a, therefore, failure to respond to androgen receptor-inhibitor chemotherapeutics.
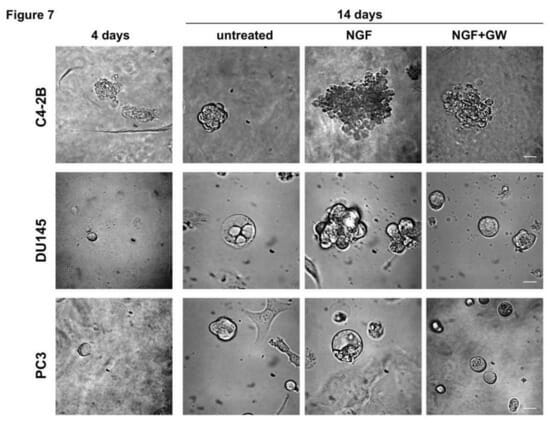
A recent study conducted by Marzia Di Donato and published in the journal Cancers has offered a possible solution to this chemoresistance. Studies have found that, when bound to the receptor tyrosine kinase TrkA, its ligand, nerve growth factor (NGF), activates signaling that leads to cell growth and proliferation. NGF is naturally expressed by the human prostate, and its misregulation has been shown to promote carcinogenesis. Building off increasing evidence that suggests that disrupted NGF/TrkA signaling is significant for prostate cancer progression, Di Donato used VitroGel® 3D-RGD (TWG002) hydrogel from TheWell Bioscience to develop 3D organoids from three different CRPC-derived cells lines, each expressing different levels of TrkA. They exposed the organoids to NGF and then evaluated each for changes in cell migration, proliferation, and invasiveness. They observed that NGF induced a mitogenic effect in all organoids, promoting a more tumor-like phenotype than untreated cells. Conversely, incubating NGF-treated organoids with an anti-TrkA siRNA disrupted migration and invasiveness. Further analysis revealed that NGF binding to TrkA promotes autophosphorylation of residue Y490, leading to downstream activation of MAPK and AKT signaling, which promote growth and cell survival, respectively. Their results found that once these signaling cascades were active, NGF and PI3K engage in a positive feedback loop, highlighting that regulation of TrkA could disrupt this feedback and shut down the growth of CRPC cells.
Traditional 2D cell culture systems have facilitated research of cellular and molecular biology for decades, but they also have significant limitations including imprecise duplication of in vivo physiological cell-cell interactions, resulting in inaccuracies in gene expression. Moreover, mounting studies show that interactions between tumor cells and ECM are critical for cancer progression and thus, recapitulation of this phenotype is critical to modeling drug response in vitro. Di Donato’s team was able to overcome these limitations using VitroGel system, a xeno-free tunable hydrogel system, to precisely manipulate the microenvironment according to the experiment design. The 3D environment allowed for a more accurate representation of endogenous signaling. Additionally, our versatile hydrogel scaffold allowed for effective treatment with exogenous factors including NGF and siRNAs, and the resulting organoids were easily imaged. Most notably, the 3D environment of VitroGel 3D-RGD hydrogels allowed Di Donato to demonstrate for the first time that TrkA activation promotes CRPC-derived organoids, effectively recapitulating tumor growth in vitro, and allowing for analysis of the effectiveness of TrkA siRNA on inhibiting tumor growth. Di Donato’s work with VitroGel 3D-RGD and CRPC cells provides a viable 3D model wherein biopsies of CRPC patients can be evaluated for Trk signaling disruptions, and new drugs specifically targeting this aggressive cancer can be developed, improving translational research and preclinical drug screening.
Related Product: