Application Notes
3D Printed Tumor Spheroids for Disease Modeling and Chemotherapeutic Drug Screening
Poster Presentation
TheWell Bioscience Inc.
Aragaw Gebeyehu1, Sunil Surapaneni1, John Huang2, Arindam Mondal1, Vivian Wang2, Nana-Fatima Haruna2, Peggy Arthur1, Shallu Kutlehria1, Nil Patel1, Mandip Sachdeva1
1College of Pharmacy and Pharmaceutical Sciences, Florida A&M University, Tallahassee, FL3230; 2TheWell Bioscience, Inc., North Brunswick NJ 08902
Abstract
3D bioprinting is intended for regenerative medicine and disease model development for drug discovery in-vitro. Herein, we evaluated the printability of a series of xeno-free and ready-to-use bioinks, developed from the tunable hydrogel (VitroGel®) system. We observed that Ink H4 and Ink H4-RGD hydrogel displayed excellent rheological properties among all screened samples. Ink H4-RGD was printable with less cell destructive extrusion pressure and showed more than 90% cell viability for both tumor and normal cells. Oscillatory rheological measurements of Ink H4-RGD hydrogel showed good printability with a wide temperature range of 20 to 37⁰C. The constructed cell-laden scaffold maintained its stiffness for more than 20 days of incubation at 37⁰C. NSCLC PDX (EGFR T790M) cells, which were cultured in 3D printed Ink H-RGD scaffold, showed high cell proliferation with tumor microenvironment and spheroid formation (500 μm in diameter) within 7 days. IC50 values of docetaxel, doxorubicin, and erlotinib demonstrated higher resistance in 3D spheroids of NSCLC-PDX, MDA MB231 WT and HCC B-27 cells when compared to 2D monolayers cells, as analyzed by cytotoxicity assay (P <0.001). Our results of the rheological analysis, shape fidelity, scaffold stability and biocompatibility of Ink H4-RGD suggest that it could be considered for cell printing and soft tissue development for high throughput screening of various anti-cancer drugs.
Materials and Methods
Rheology Properties of Bioinks: Ink-H4 and Ink-H4-RGD were used in this the rheological test. Two groups of samples were prepared: group 1) hydrogel without mixing with cell culture medium; group 2) hydrogel mixed with DMEM-F12 medium at 5:1 ratio.
Printability of Bioinks: Inks H1(S1), H1-RGD (S2), H2(S3), H2-RGD (S4), H3(S5), H4(S6), H4-RGD (S7) and H5 RGD (S8) were used for printability and evaluation of scaffold formation. Hydrogels were loaded into 3 mL printing cartridges and connected to a controllable pressure regulator on Bio-X printer. Three-layered grids were printed on a petri-dish using 22G nozzle and 25G nozzle and the printability was evaluated in terms of shape fidelity, applied extrusion pressure, and post-printing cell viability.
Cell Printing: For each cell line, 400 μL of cell suspension (5 x106 cells) was mixed homogenously in 2 mL of S7 (Ink H4 -RGD) bioink by a sterile spatula and loaded carefully into the printer cartridge and printed using 22G nozzle. After printing, each cell line was cover with 500 μL of Organoid cell culture medium. The cell viability was evaluated immediately after gel mixing before printing, immediately after printing, on the 2nd, 4th, 7thand 10thdays of post-printing.
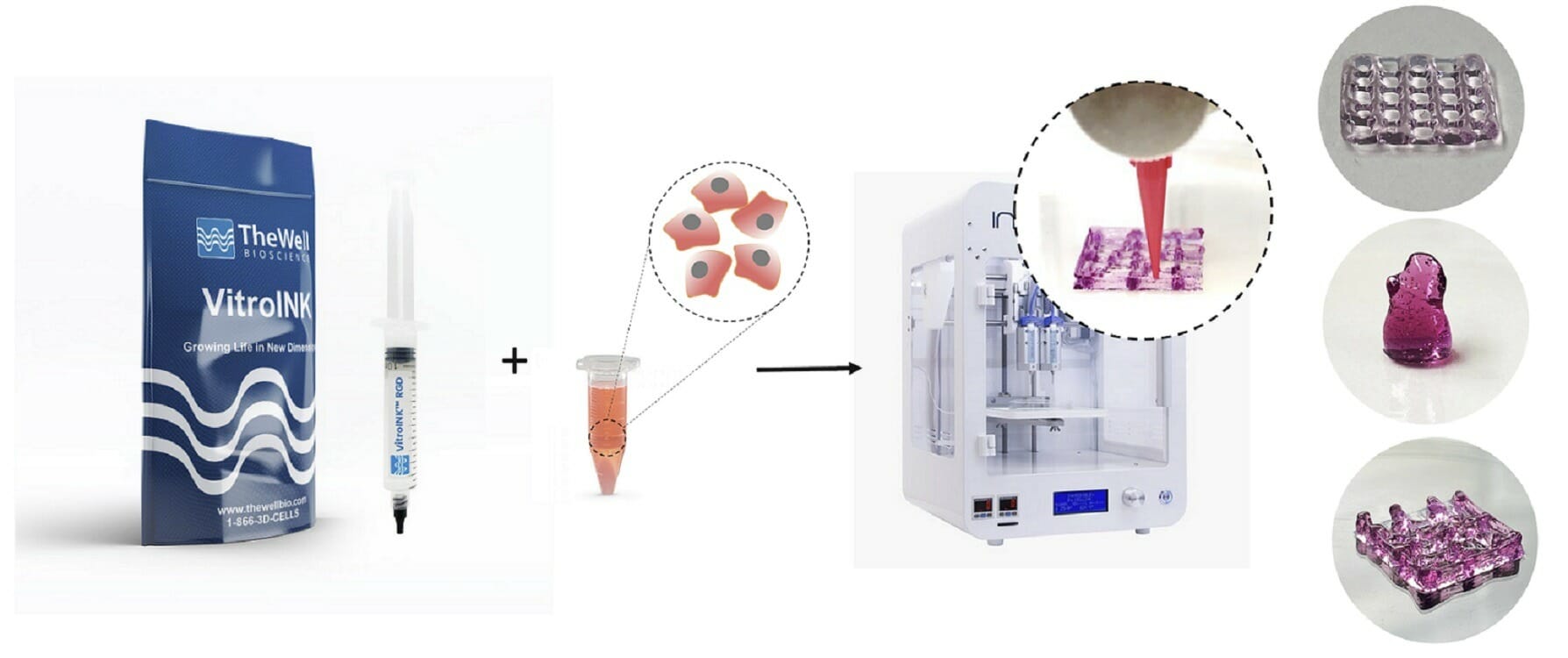
VitroINK™ is a series of ready-to-use bioinks, that can print without UV or heat curing or chemical cross-linking. The VitroINKs are room temperature stable. Cells can be pre-mix in the hydrogel or directly mix with the bioink by using a dual syringe system. Multiple biological functional binding ligands/components can incorporate with this bio-ink for different applications.
Rheology Properties of Bioinks
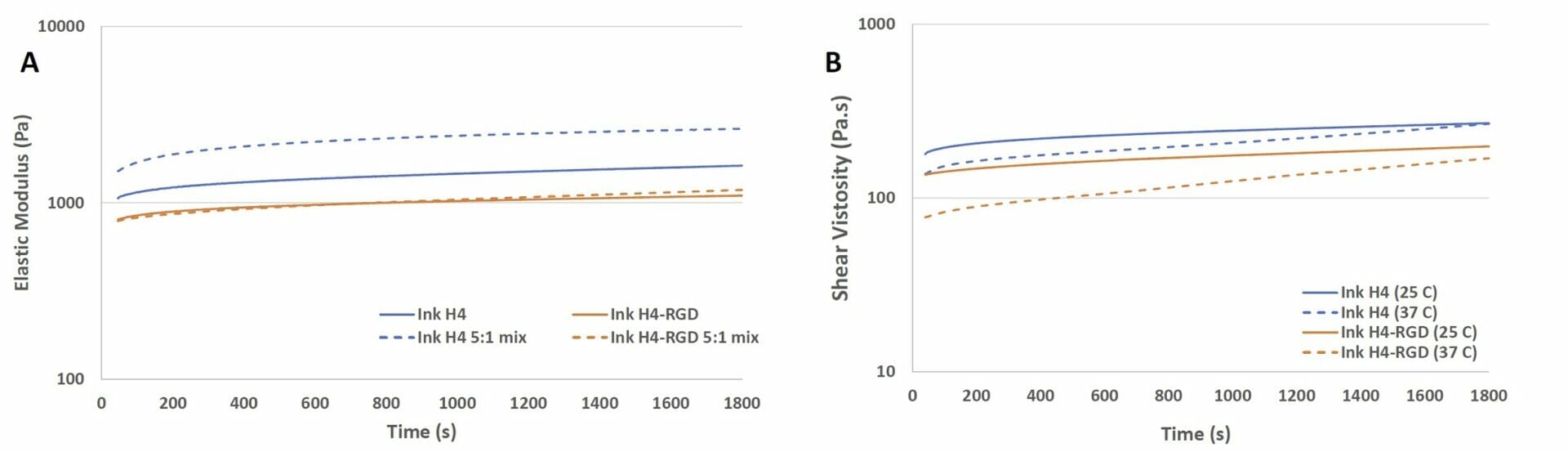
Figure 1. Elastic modulus and viscosity of Ink H4 and Ink H4-RGD under the time sweep test. A) the elastic modulus of Ink H4 and Ink H4-RGD with and without 5:1 mixing with cell culture medium; B) the viscosity of Ink H4 and Ink H4-RGD at 25 and 37°C.
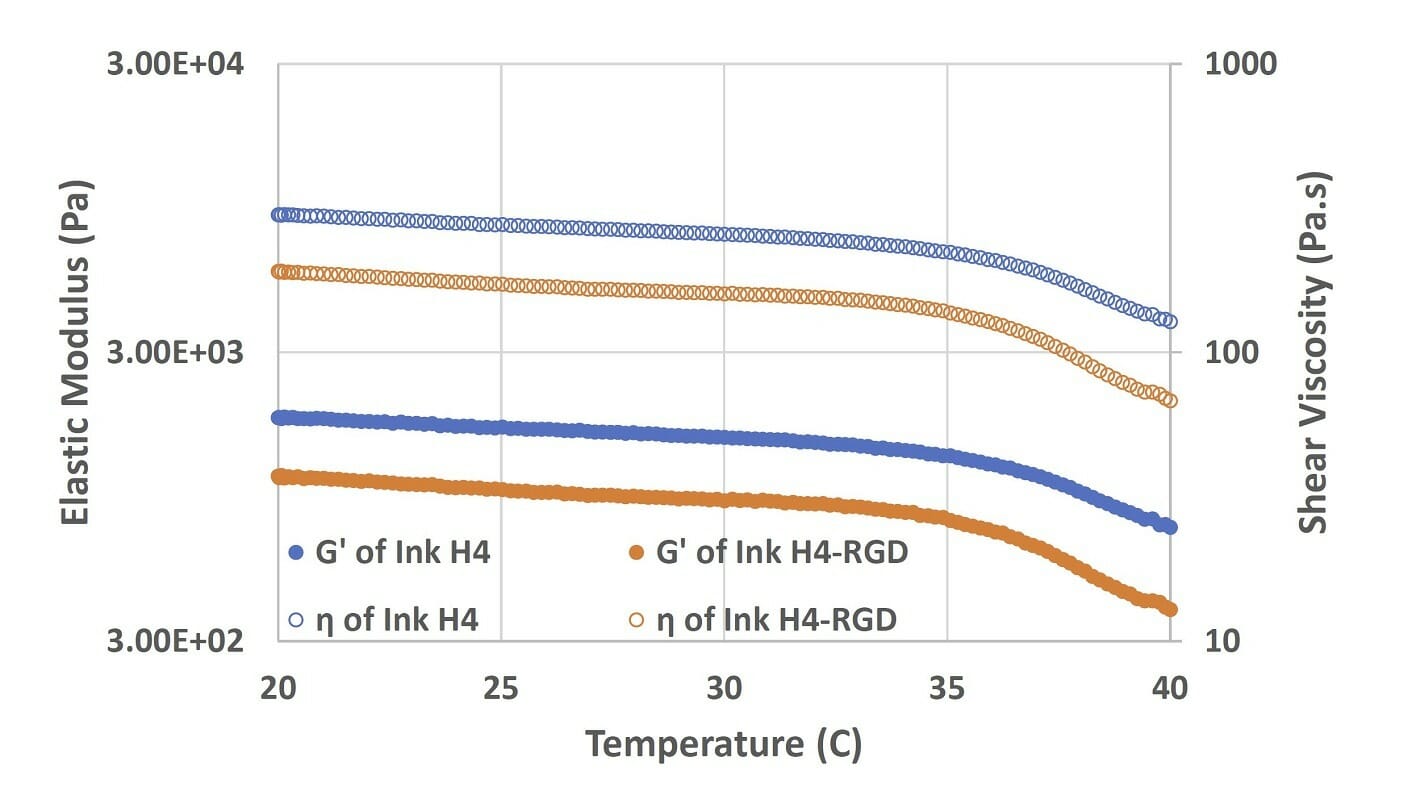
Figure 2. Elastic modulus and viscosity of Ink H4 and Ink H4-RGD under the temperature sweep test (from 20 to 40°C).
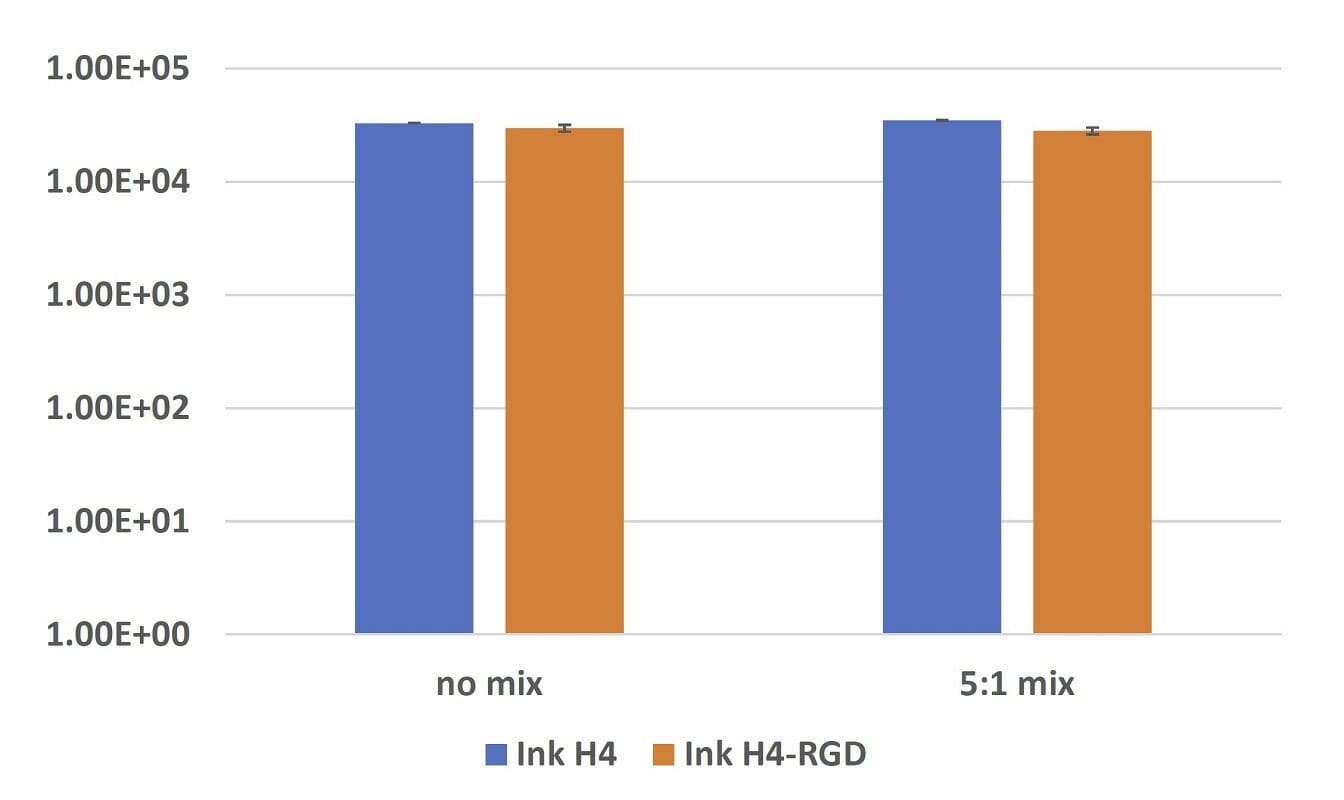
Figure 3. Elastic modulus of submerged Ink H4 and Ink H4-RGD samples with or without 5:1 mixing with cell culture after 24-hours incubation.
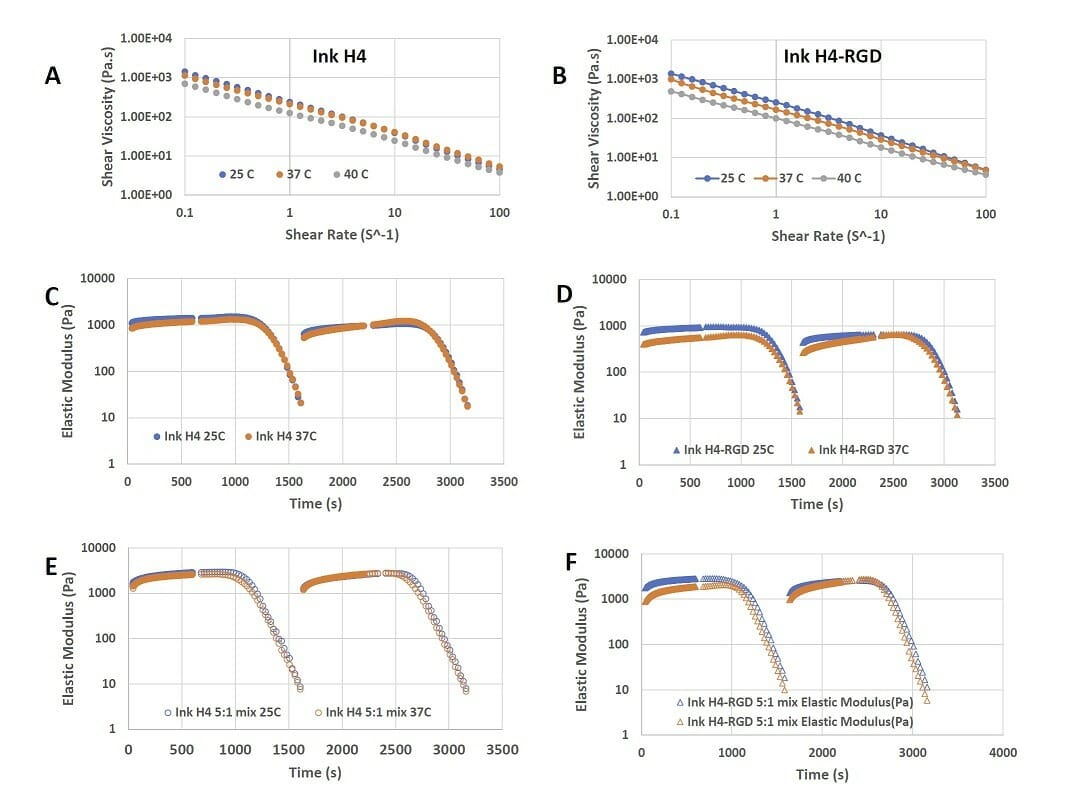
Figure 4. Shear-thinning and rapid recovery property of Ink H4 and Ink H4-RGD. A) the shear-thinning of Ink H4 at 0.1 to 100% shear strain at 25, 37, and 40°C; B) the shear-thinning of Ink H4-RGD at 0.1 to 100% shear strain at 25, 37, and 40°C; C)-F) the repeated shear-thinning and rapid recovery properties of Ink H4 and Ink H4-RGD with or without 5:1 mixing with cell culture at both 25 and 37°C.
Printability & Bioink Cytocompatibility
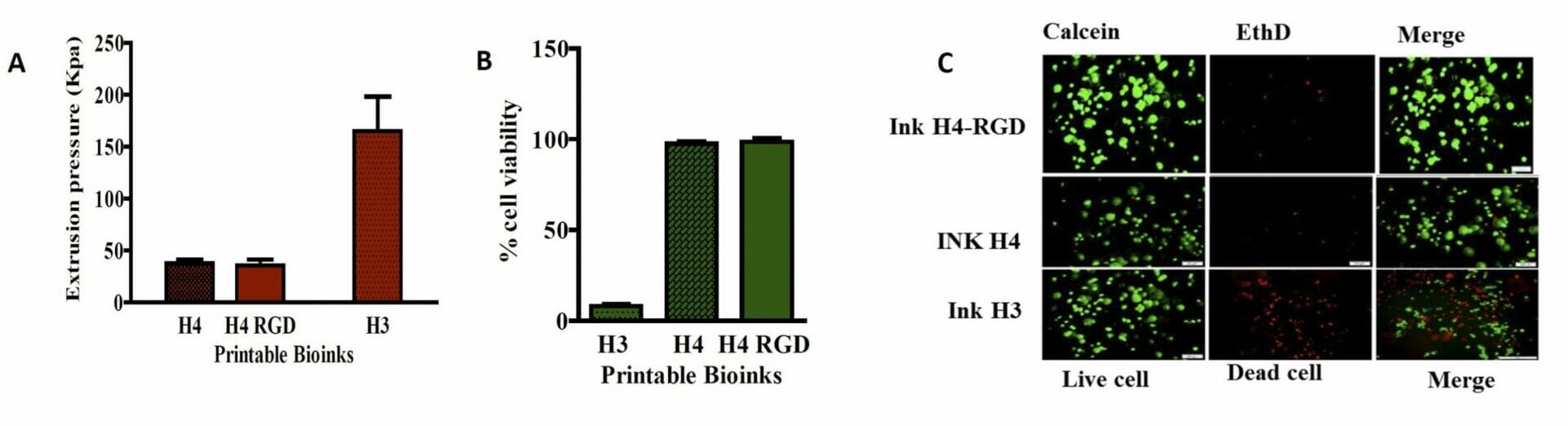
Figure 5. A) Extrusion pressure required for printing of bioinks B) Cell viability assay in MDA-MB-231 Wtcells C) Live/dead cell assay analysis using CalceinAM/Ethidium homodimer III staining. Results shown were representative of three independent experiments.
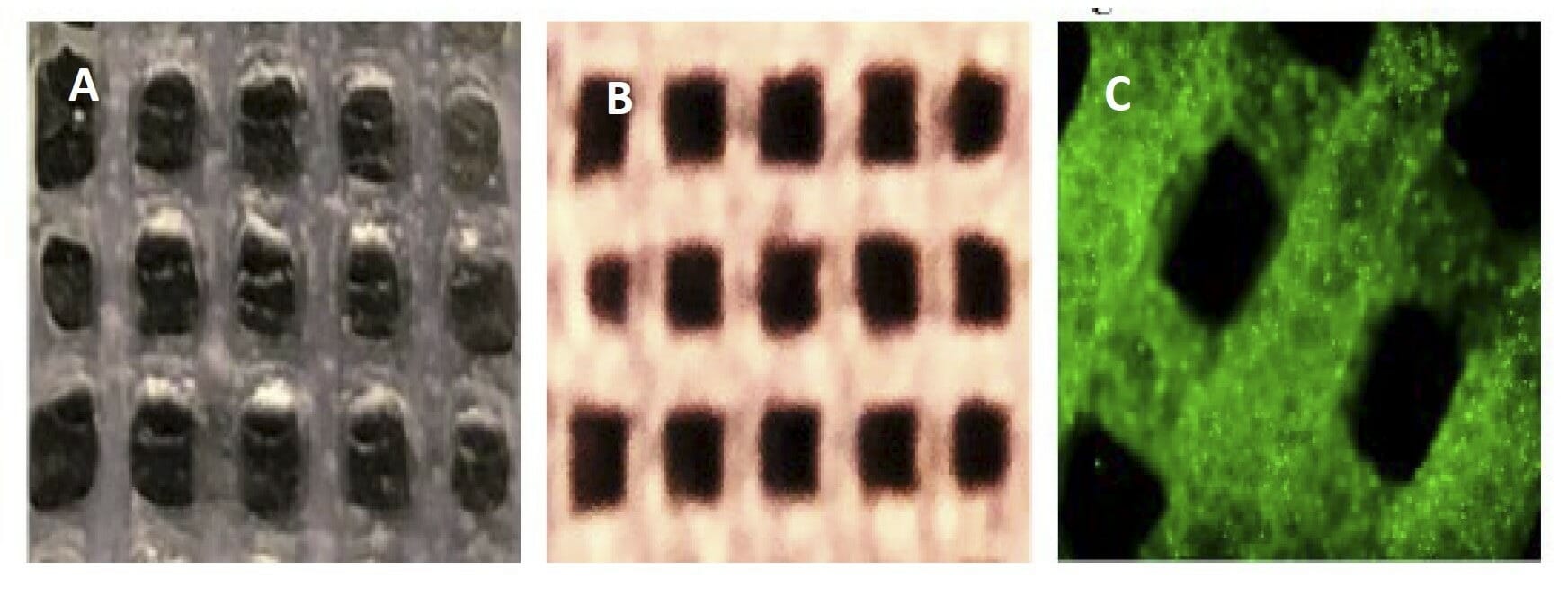
Figure 6. A) Photo image of a partially crosslinked scaffold of Ink H4-RGD B) Photo image of fully a crosslinked scaffold of Ink H4-RGD C) Microscopic image of cell (PDX-NSCLC) laden Ink H4-RGD scaffold.
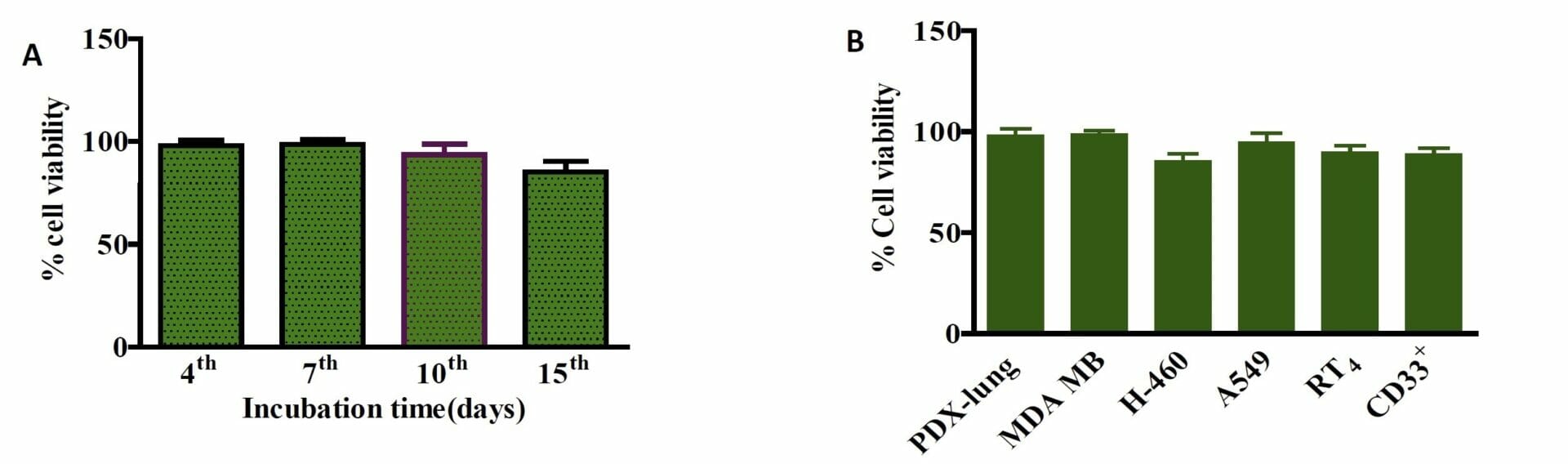
Figure 7. Biocompatibility of Ink H4-RGD. A) PDX-NSCLC cells viability when encapsulated with ink H4-RGD for 15 days B) Ink H4-RGD biocompatibility in various cancer cells. Results shown were representative of three independent experiments.
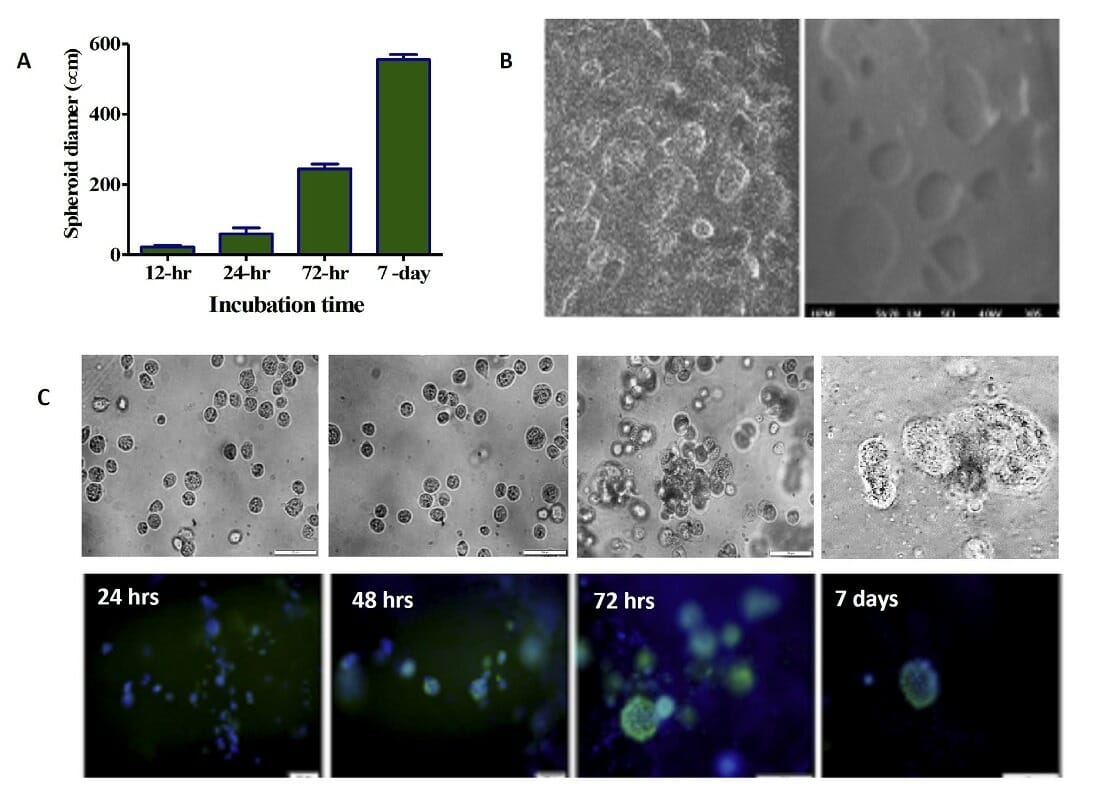
Figure 8. 3D printed cells/spheroids growth in printed ink H4-RGD scaffold. A) Average spheroid proliferation/growth rate over a period of 7 days B) SEM images of spheroids inside the ink H4-RGD scaffold C) Spheroid images under bright field microscopy and after staining with NucBlue/ Actin Green staining over a period of 7 days
Anticancer Drug Sensitivity Evaluation
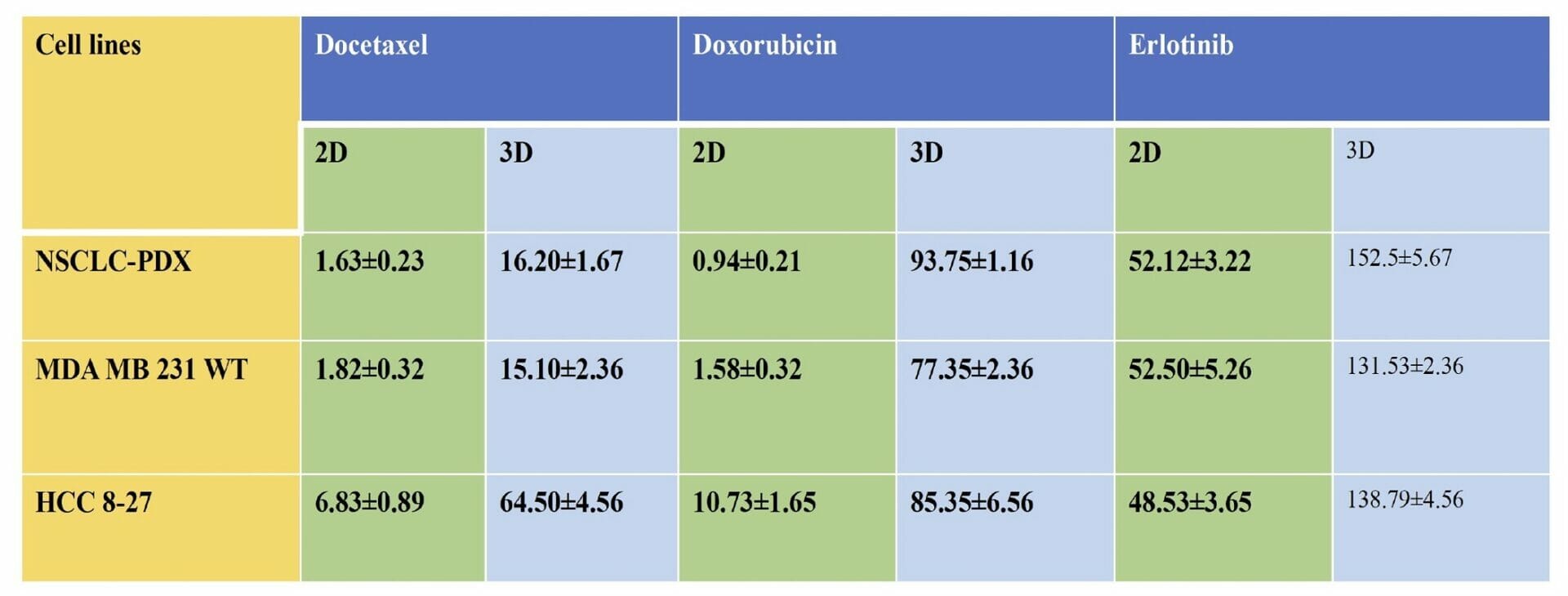
Table 1. Cytotoxicity (IC50 values ) of Docetaxel, Erlotinib and Doxorubicin in 2D monolayers and 3D spheroids of MDA-MB-231 WT, PDX-NSCLC and HCC-B27 cells. The IC50 values in 3D printed cell spheroids ranged from 3 to 90 folds higher than the 2D counterparts with equivalent cell numbers, all these suggest that tumor complexity contributes to decreased sensitivity of docetaxel, erlotinib and doxorubicin in 3D spheroids.
- VitroINK presents promising properties as a bioink system in terms of printability, shape fidelity, rheological parameters, scaffolds morphology, cell viability and rapid spheroid formation.
- Directly using cell culture medium for printed scaffold stability, the VitroINK system could be beneficial for cell growth and 3D cell scaffold within vivo stromal characteristics.
- 3D spheroids have shown more resistance to doxorubicin, docetaxel and erlotinib in comparison to 2D monolayers of NSCLC PDX, MDA-MB-231 WT and HCC-B27 cells.


