Research Highlights
Defrosting Fertility: VitroGel offers a viable alternative to ovarian tissue cryopreservation
In vitro three-dimensional culture of murine ovarian follicles in the VitroGel demonstrates superior morphology and growth compared to other biomaterials and 2D culture.
Institution:
Seoul National University, Eulji University
Team:
Eun Jung Kim, Chungmo Yang, Jaewang Lee, Hye Won Youm, Jung Ryeol Lee *, Chang Suk Suh, Seok Hyun Kim
Application:
3D follicle culture
Disease model:
In vitro follicle culture, Fertility preservation
Cell types:
Ovarian follicles isolated from mice
Hydrogel:
VitroGel® RGD (TWG003)
Cancer treatment can adversely affect fertility in a number of ways, from ovum destruction resulting from chemotherapeutic treatment or radiation therapy to adverse effects of hormone secretion or function in response to brain treatment, the reproductive organ cancers necessitating removal of all or part of the ovaries or uterus. Accordingly, there are a number of fertility treatment options available, the most popular being egg or embryo freezing. While this procedure, in general, has certain limitations including potential damage of eggs during freezing and thawing, the greatest limitation is that the required process of hyper-ovulation takes 10 days, making this unfeasible for patients needing immediate treatment. To address this, a more experimental alternative known as ovarian tissue freezing has made headway as a more immediate form of fertility preservation. This process involves removing an entire ovary and freezing pieces of the tissue for future reimplantation. However, recent studies have raised some concerns over this method, namely for women diagnosed with ovarian or uterine cancer, including the possibility of transplanting malignant cells back into patients who are in remission, necessitating another alternative. One such proposed alternative is ovarian follicle isolation combined with in vitro culture, but this method has been limited due to challenges in promoting follicle growth and maturation that parallels in vivo development.
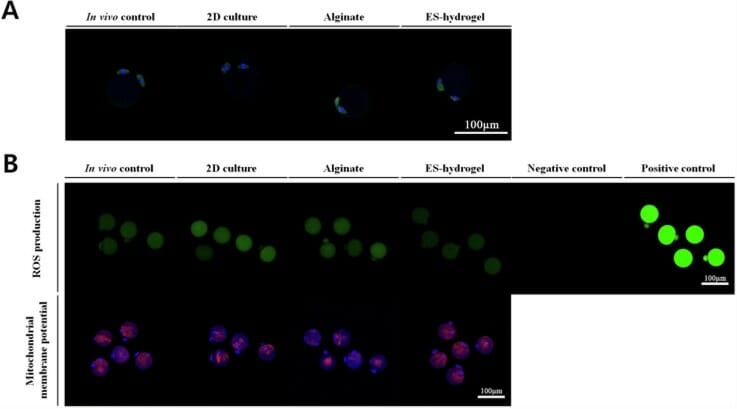
Current 2D culture systems for follicle culture produced live mice in 1996, but recapitulation with human ovarian follicles resulted in the collapse of the follicular structure and overall morphology due to spreading on the surface of the culture dish. Consequently, studies have turned to 3D biomaterials, but ongoing studies have run into roadblocks because it has been difficult to generate a scaffold that meets all the needs of the developing follicles without damaging them. Though some studies have various types of hydrogels with some level of success in follicle encapsulation, the xeno-free tunable VitroGel system has not been evaluated in this application. Therefore, Eun Jun Kim and colleagues conducted a study to evaluate the success of VitroGel RGD in addressing these issues for successful 3D follicle culture. Using mouse follicles, they compared VitroGel to the commonly used alginate scaffold, assaying for morphological changes and the ability of the follicles to grow and mature. In a side-by-side comparison using the same isolation and culture protocols, they found that while both 3D culture options fared better than traditional 2D culture, the softer VitroGel RGD hydrogel significantly outperformed the alginate scaffold. Specifically, follicles encapsulated in VitroGel demonstrated a more efficient rate of maturation as evidenced by the number of follicles developing the pseudo-antrum, and better follicle survival, ultimately yielding significantly more retrieved mature oocytes than were retrieved from the alginate scaffold. Moreover, mature oocytes retrieved from the hydrogels exhibited higher rates of normal chromosomes and mitotic spindle formation than those isolated from the alginate hydrogel. The tunable VitroGel system gives the flexibility to adjust the mechanical properties of the scaffold from soft to hard, which leads to a successful optimization in follicle culture.
These studies, though preliminary, highlight the great potential for VitroGel system in reproductive applications. Another critical difference between these scaffolds is the means by which the follicles were retrieved. In order to retrieve the embedded follicles in both hydrogels, scaffolds must be dissolved. The alginate scaffold requires treatment with the enzyme alginate lyase, which has been shown to be damaging to the follicle’s basement membrane and theca cells, whereas VitroGel only requires phosphate-buffered saline, a cell isotonic solution that causes no harm to cells.
Though these studies are preliminary and will also require evaluation of human follicular cells and other follow-up studies, this study highlights the great potential of VitroGel system for reproductive applications.
Related Product: