Research Highlights
VitroGel’s ability to enhance the ECM-cell interaction enhances transplantable capacity of MSCs in rat models of intervertebral disc regeneration
VitroGel® RGD enhances stem cell transplantation to treat intervertebral disc degeneration using rat models, laying new foundations in the movement toward cell-based tissue therapies.
Institution:
Dalian Medical University, Clinical Medical College of Yangzhou University
Team:
Feng Wang, Li-ping Nan, Shi-feng Zhou, Yang Liu, Ze-yu Wang, Jing-cheng Wang, Xin-min Feng *, and Liang Zhang
Application:
3D culture for transplantation studies
Disease model:
Intervertebral disc degeneration
Cell types:
NPMSC
Hydrogel:
VitroGel® RGD (TWG003)
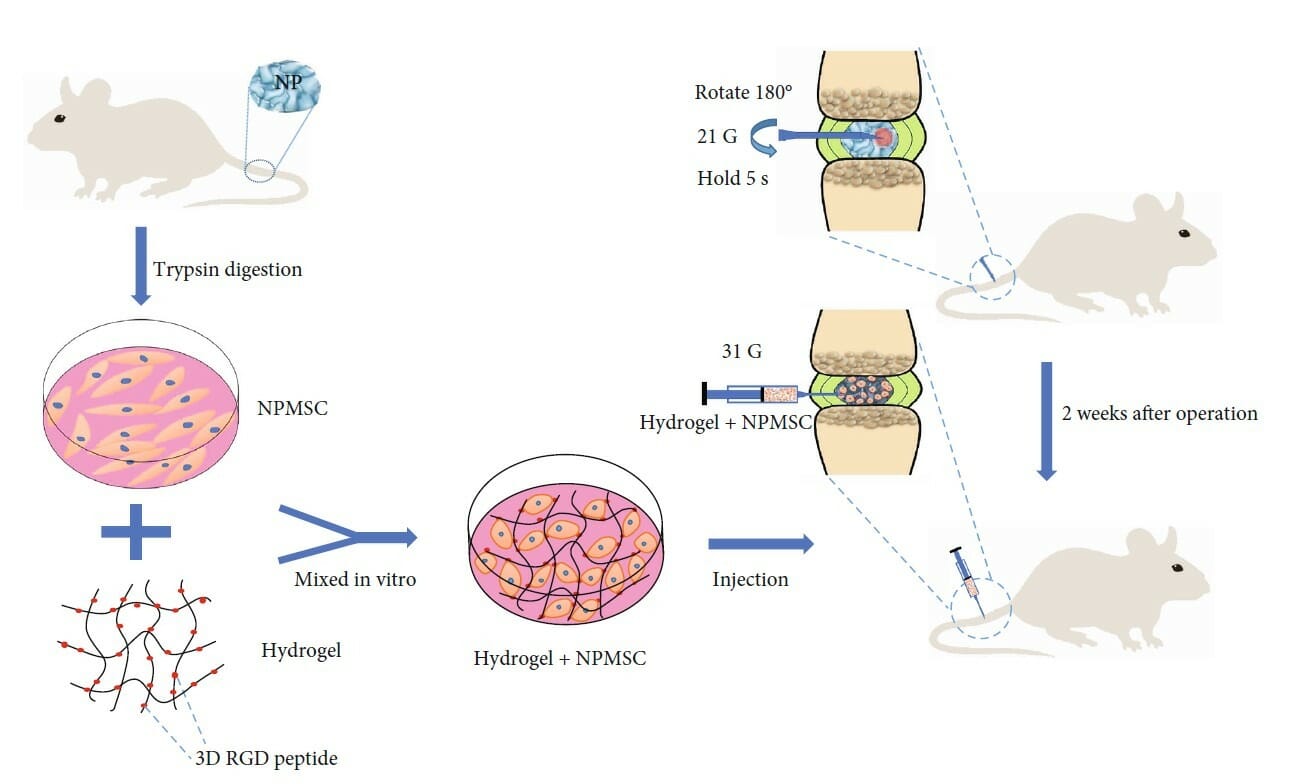
Lower back pain caused by intervertebral disc degeneration is becoming increasingly common due to the growing aging population. However, there exists no common therapy that can effectively repair or regenerate the structure and function of degenerative discs, necessitating the development of a new treatment approach. Intervertebral discs are composed of three major parts, the most critical of which is thought to be the central gelatinous nucleus pulposus. This part is thought to have the most suitable extracellular environment for the growth and secretion required for maintenance and is therefore believed to be of great importance to the treatment of intervertebral disc degeneration. Several previous studies have demonstrated that mesenchymal stem cells (MSCs) show great promise in addressing this problem, but the survival of transplanted MSCs is a major obstacle for transplantation therapy, indicating that cell transplantation alone cannot repair the local degenerated microenvironment. Therefore, transplantation of cells embedded in scaffolds that mimic the local microenvironment is has become the ideal approach for the treatment of intervertebral disc degeneration. Given the broad array of hydrogels available, each with broad rheological parameters, a recent study by Wang and colleagues sought to identify an ideal hydrogel that was ideal for this application. Using MSCs derived from the nucleus pulpous (npMSCs), which have recently been shown to exhibit superior proliferation and ability to adapt to the intervertebral disc microenvironment, they evaluated the ability of the tunable, injectable RGD-conjugated VitroGel hydrogel scaffold to address intervertebral disc degeneration in a rat model. Initial in vitro demonstrated that npMSCs embedded into VitroGel were capable of differentiating into mature nuclear pulpous-cytes, were capable of activating other endogenous cells, and were capable of triggering an anti-inflammatory response. With histological staining, they confirmed that rats with disc degeneration that were transplanted with the npMSCs-VitroGel composite presented with more regular disc structure and better extracellular matrix (ECM). Immunofluorescence and gene expression analysis confirmed that the expressions of factors critical in the regeneration of disc tissue were significantly higher than untreated groups and exhibited better overall survival over a period of 30 days than cells transplanted without VitroGel. Interestingly, Wang and colleagues noted that the hydrogel alone did not promote tissue regeneration, suggesting that the hydrogel enhances the regenerative effect of the transplanted npMSCs by promoting ECM synthesis, a specialty of VitroGel RGD, the RGD peptide conjugated hydrogels.
The experimental duration in this study needs to extend to reflect the complete effect of the transplantation of npMSC-VitroGel treatment on the repair of degenerative tissue; effective treatments will need to be stable and functional for much longer periods of time and facilitate true regeneration to be considered as a serious therapeutic option. Though preliminary, this study has successfully demonstrated that the tunable, injectable VitroGel hydrogel conjugated with the RGD peptide is a promising and viable way to help promote the proliferation, differentiation, and survival of npMSCs in vitro and in vivo. This study has laid the groundwork for ongoing studies using injectable VitroGel in regenerative studies, both in intervertebral disc degeneration and other studies that rely heavily on proper ECM-cell interaction.
Link to full paper:

