Research Highlights
Silent Partner: The MYC-related pseudogene HMGA1P6 drives malignancy in ovarian cancer
In vitro three-dimensional culture helps to demonstrate that several pseudogenes, including HMGA1P6, are involved in metastasis of ovarian cancer, introducing another unconventional target for cancer therapeutics.
Institution:

Shandong University
Team:
Xiaoxue Tian, Jianping Song, Xiyu Zhang, Mingyao Yan, Shourong Wang, Yuqiong Wang, Limei Xu, Ling Zhao, Jian-jun Wei, Changshun Shao, Beihua Kong *, and Zhaojian Liu *
Application:
3D tissue model to evaluate the role of a gene in tumor progression
Disease model:
Ovarian cancer
Cell types:
HO8910, HEK293T, A2780,HEY, SKOV3 cell lines
Hydrogel:
VitroGel® RGD (TWG003)
Ovarian cancer has the highest mortality of all gynecologic cancers and is often diagnosed at an advanced stage as there exist no effective screening protocols for earlier diagnosis. Moreover, following diagnosis, the 10-year survival is below 30% and has not been improved significantly over the last 30 years since currently available targeted therapies demonstrate inconsistent and transient patient responses. Further contributing to the poor progression of treatment and diagnosis is the fact that the mechanisms that regulate ovarian cancer progression and recurrence remain poorly characterized.
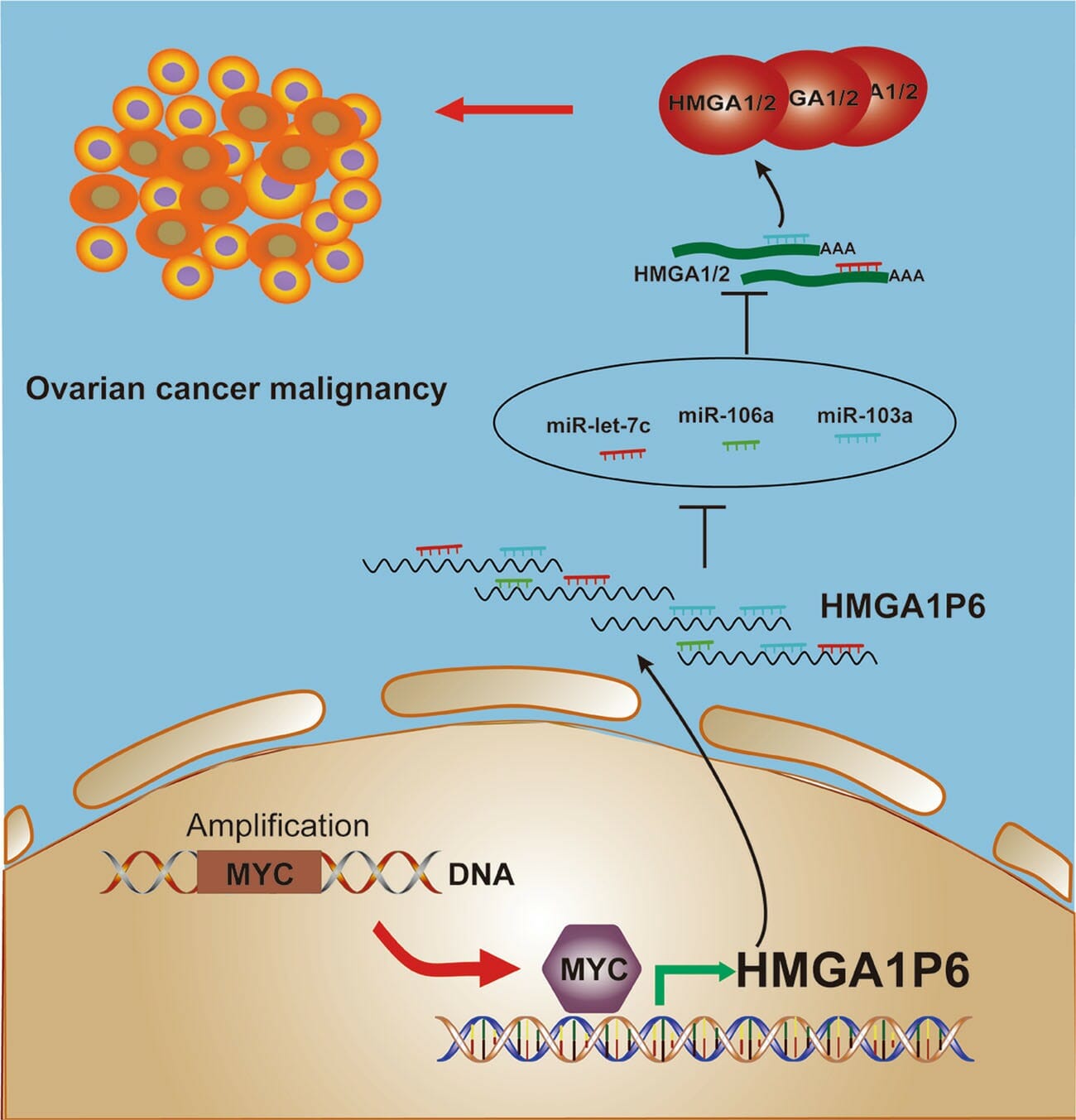
Pseudogenes are nonfunctional segments of DNA with structure akin to coding genes, often arising as defunct copies of actual functional genes. These gene species are relatively common, with roughly 10% of functioning genes having pseudo targets, though they have long been touted as ‘junk DNA’ but increasing scientific evidence suggests that pseudogenes have a certain level of functionality, both transcriptionally and post-transcriptionally. Though pseudogenes have been implicated in other cancers, the role of pseudogenes in ovarian cancer remains unclear. A recent study by Xiaoxue Tian and colleagues at Shandong University sought to identify the differentially expressed pseudogenes in ovarian cancer patients. Their study evaluated several overexpressed pseudogenes but focused on HMGA1P6 as one of the more highly expressed pseudogenes. Their study found that HMGA1P6 was associated with ovarian cancer aggressiveness through modulating HMGA1/2, and more importantly, that HMGA1P6 is a direct transcriptional target of MYC, one of the most frequently amplified genes in multiple cancers, especially ovarian cancer. HMGA1P6 is a pseudogene of HMGA1 and produces a long noncoding RNA that has been shown to compete with endogenous RNAs in other tumors. HMGA1 is a nonhistone chromatin remodeling proteins normally expressed at high levels during embryogenesis, but low or absent in most adult tissues. They found that HMGA1P6 regulates HMGA1 as competing endogenous RNA leading to enhanced HMGA1 and HMGA2 expression. They also found that HMGA1P6 was transcriptionally activated by oncogene MYC in ovarian cancer patients, suggesting that malignancy is driven by HMGA1P6 through indirect activation by MYC.
In this study, Tian and colleagues used tunable 3D environment generated by TheWell Bioscience’s VitroGel RGD to mimic the tumor microenvironment in order to evaluate the role of HMGA1P6 in ovarian tumor progression. Various studies have shown that tumor cells cultured two-dimensional environments exhibit distinct differences in cell proliferation capacity, differentiation ability, invasive ability, etc., highlighting the importance of the appropriate environment in accurate elucidation of genes in tumor behavior. VitroGel provides that 3D environment that not only provides the proper environment for gene function evaluation but the tunable and flexible nature of the hydrogel sets up the possibility for continuity between the current studies and future drug and other treatment studies.
Tian and colleagues demonstrated that pseudogene HMGA1P6 competitively regulated histone modifiers HMGA1/2 in a 3D in vitro tumor model, introducing HMGA1P6 as a valuable prognostic marker and promising therapeutic target for ovarian cancer.
Related Product: