Application Notes
Using the VitroGel® Hydrogel System as a Versatile Platform for Invasion Assay
Application Note
Nana-Fatima Haruna, Atsuo Ochi, John Huang
TheWell Bioscience, North Brunswick, NJ 08902
Introduction
Invasion studies have been crucial in identifying key intracellular signaling pathways and membrane-bound and soluble enzymes that influence cellular movement through a three dimensional (3D) extracellular matrix (ECM)1,2. Such information has been used to investigate mechanisms governing the infiltration of tumor cells into healthy tissue, ultimately leading to the discovery of novel drug targets. Additionally, invasion assays have aided in the development of compounds targeting tumor invasion and metastasis.
While two-dimensional (2D) invasion models have provided some insight, recent studies have illustrated that they do not accurately recapitulate natural ECM structure and composition. Therefore, 2D systems possess limitations in producing clinically relevant data for cancer drug discovery3,4. Since tumor progression is highly dependent on cell-cell and cell-matrix interactions, 3D environments more accurately and effectively model tumor growth and metastasis. However, while 3D environments provide an excellent tumor modeling system, invasion assays using animal-derived basement membrane matrices, such as Matrigel, limit in-depth examination of the various biochemical and biophysical factors that regulate tumor formation, progression, and metastasis. Myriad factors hinder the application of animal-derived matrices in cancer studies. First, these matrices contain unknown concentrations of important ECM bioactive ligands. Second, the inability to easily adjust mechanical of animal-derived gels inhibits an in-depth exploration of the influence of mechanical forces on tumor invasion and metastasis and biochemical properties and specific molecules (e.g. binding ligands, chemokines, and growth factors) on tumor invasion and metastasis5,6. Third, animal-derived hydrogels often contain non-human growth factors and hormones in unknown concentrations.
Xeno-free synthetic hydrogels, such as VitroGel, offer an excellent platform to overcome the limitation of animal-derived hydrogels. VitroGel provides a highly consistent 3D hydrogel system that closely mimics the in vivo ECM. Native ECM hydrogels contain undefined concentrations of functional ligands, which can have important, unpredictable effects on cancer cell proliferation, morphology, migration, and invasion. Conversely, VitroGel hydrogels contain defined, and consistent, concentrations of functional ligands that may affect tumor development and metastasis. For example, VitroGel RGD is modified with an RGD peptide, the primary integrin-binding domain of several ECM proteins. RGD can promote cancer cell adhesion7, spreading8, migration7, proliferation9, and spheroid formation9. The ECM mechanical properties are another factor that highly regulates tumor growth and metastasis. In fact, ECM density and rigidity affect the cancer cell invasive-like activity10 and migration velocity11. While these studies have found substrata dependent cancer cell activity, the effects of ECM density and stiffness remain somewhat controversial, due to contradictory results. Therefore, a highly adjustable system like VitroGel allows researchers the ability to tease apart the intricacies of stiffness dependent cancer cell invasion. Finally, another critical factor in tumor growth and metastasis is chemoattractant signaling. For example, CXCL12, a ubiquitously expressed chemokine that directs cell migration under normal conditions, has been shown to induce proliferation and invasion of human cancer cells12. Other growth factors, such as epidermal growth factor and platelet-derived growth factor, have been shown to modulate cancer cell behavior13. Therefore, the presence of growth factors in animal-derived matrices may have important and confounding effects on cancer development and growth. However, VitroGel offers a system in which cancer cells may be exposed to growth factors in a well-defined manner.
Here, we investigated the ability of our xeno-free hydrogel, VitroGel, to test 3D cancer cell invasion. Additionally, we evaluated the role of mechanical stress on invasive cellular behavior. The tunable and functional hydrogel system can be manipulated to study many different factors that may influence cell invasion and mobility, such as mechanical strength, ECM degradation, varying functional ligands, and serums/growth factors/cytokines/chemokines. Invasion assays can be performed in different ways in this system including XY and XYZ cell migration and 3D spheroid invasion. In this study, we focused on methods designed to measure vertical invasion of cancer cells using both a Transwell insert and 2D hydrogel coating. Specifically, we monitor the vertical movement of Glioblastoma U87 MG and neuroblastoma B35 cells through the hydrogel matrix over time. These cancer cell subtypes are known to be highly invasive and metastatic14,15. We explored the effects of an integrin functional ligand, RGD, and a chemokine, CXCL12, on tumor cell invasion.
Materials and Methods
Cell Culture
U87-MG cells were maintained in Alpha Minimum Essential Medium supplemented with 10% FBS. B35 cells were maintained in Dulbecco’s Modified Eagle Medium supplemented with 10% FBS. The cells were passaged when the cultures reached 80-90% confluence.
Invasion Assay with Cell Culture Insert
Transwell inserts (8 μm pore size) were placed in 24 well plates and surfaces were coated with either VitroGel 3D or VitroGel RGD according to the 2D hydrogel coating protocol in the user handbook, as described below:
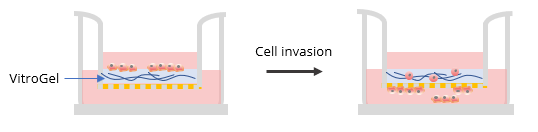
Two ratios were used in this experiment: a) Dilution ratio (the ratio of the hydrogel solution to Dilution solution), and b) Mixing ratio (the ratio of the diluted hydrogel solution to cell culture medium). To study the effects of FBS, human platelet lysate, integrin-binding ligands, and hydrogel mechanical properties on cell invasion, both VitroGel 3D and VitroGel RGD were diluted with VitroGel Dilution Solution (Type 2) at ratios of 1:3, 1:5, or 1:10 (dilution ratio, v/v). The diluted hydrogel solution was then mixed with cell culture medium at a 4:1 (mixing ratio, v/v) ratio. In the hydrogel mixture, final FBS concentrations of 0% and 2% were used for experiments with U87-MG cells and B35 cells, respectively. The hydrogel mixture (100 μL) was then added to a 24-well plate Transwell insert. The plate was incubated at room temperature for 30 min (1:3 or 1:5 dilution ratio) and then moved to a 4°C refrigerator, where it was incubated overnight. Afterward, the plate was brought to room temperature and 50-100 μL of cells (0.5-1 x 106 cells/mL) suspended in medium with 0% FBS (B35) or 1% FBS (U87-MG) were added to the top of the hydrogel (option: add 100 μL cell culture medium on top of the hydrogel and incubate at room temperature for 2 hours before adding cells). Next, 800 μL of cell culture medium with 20% FBS (B35) or cell culture medium with 1% FBS and 2% human platelet lysate (U87-MG) was added into the outside well of the Transwell insert. The experiment set up is illustrated in the diagram below.
Invasion Assay Without Cell Culture Insert (in a Well plate)
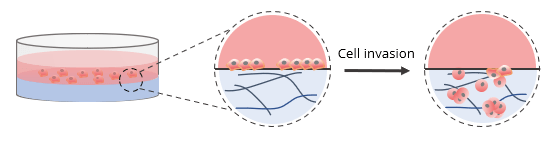
For invasion assays without a cell culture insert, 2D coated hydrogels were prepared by mixing VitroGel RGD at a 1:3 (v/v) dilution ratio with VitroGel Dilution Solution (Type 2). The diluted hydrogel was then mixed with αMEM + FBS at a 4:1 mixing ratio (v/v). This yielded a final FBS concentration of 10% in the hydrogel mixture. In a 96-well plate, 50 μL of the hydrogel mixture was added to a single well. The plate was incubated at room temperature for 1-2 hours to allow for gel stabilization. Afterward, 50 μL of U87-MG cells (0.5 x 106 cells/mL) were added to the top of the hydrogel. The cells were suspended in serum-free medium to promote cell attachment and invasion into the hydrogel matrix by following an FBS concentration gradient. This procedure is shown in the diagram below.
Cell Imaging
For cells cultured with on a Transwell insert, we observed cell localization in the 3D environment every day. On day 2 (for U87-MG cell) and day 7 (for B35 cells), the cell-hydrogel mixture on the top of the insert well was removed with cotton swabs and cells attached to the bottom of the insert well were fixed with 4% formaldehyde for 10 min, followed by permeabilization for 2 min with 100% Met-OH and staining with crystal violet for 15 min. Images were taken using a Zeiss Axiovert 200M Fluorescence cell Imaging Microscope with ZEN software.
For 2D coating hydrogel cultures, cells cultured in a 96 well plate were stained with calcein AM, a green fluorescent probe for living cells. XYZ stack images of the 2D hydrogels were obtained on days 1 and 3 to monitor cell movement on XYZ plane. The images were obtained using ImageXpress Nano (Molecular Devices) at 10X magnification
Results
Effects of FBS, human platelet lysate, integrin-binding ligands, and the hydrogel mechanical properties on cell invasion
To evaluate the advantages of VitroGel for invasion assays, the RGD modified hydrogel (VitroGel RGD) was used to induce cell invasion. As a negative control, we used unmodified Vitrogel 3D. An FBS gradient was created to promote cell invasion through the hydrogel matrix. As shown in Figure 1 b & c, after 7 days in culture, more B35 cells successfully invaded through VitroGel RGD to the bottom of the insert than through VitroGel 3D (fig 1a). This result indicates that the integrin-binding RGD ligand permits 3D invasion of neuroblastoma cells through a matrix toward an attractive cue, i.e., FBS. Some of the cells at the bottom of the Transwell insert also migrated to the bottom of the well plate (fig. 1c). Additionally, the RGD binding ligand supported neuroblastoma spheroid growth inside the hydrogel matrix (fig. 1d).
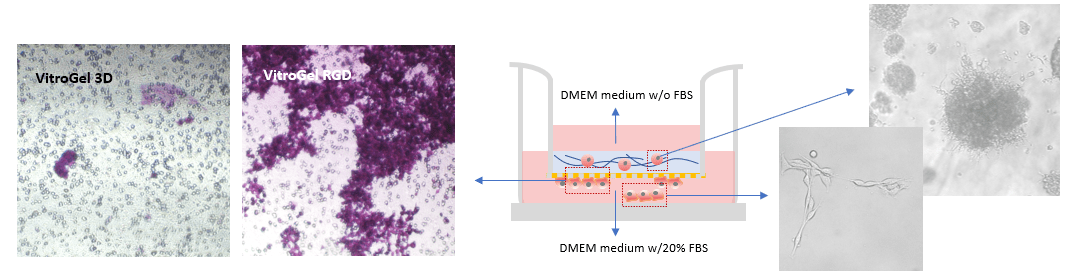
Figure 1: Comparison of B35 neuroblastoma cell invasion through VitroGel 3D (a) and VitroGel RGD (b) shows that the invasion pattern is more evident with RGD present. The hydrogel was prepared at a 1:3 dilution ratio with 2% FBS while the medium outside the Transwell insert contained 20% FBS. Cells at the bottom of the well plate (c) and cells that remained within the RGD hydrogel matrix (d) were imaged on day 7.
The effect of the integrin-binding ligand was also evaluated using U87-MG glioblastoma cells on hydrogel samples with different dilution ratios (1:5 and 1:10). Figure 2 shows that, in both dilutions, more cells migrate to the bottom of the Transwell insert through the hydrogel modified with RGD (fig. 2 c & d) than the unmodified hydrogel (fig. 2 a & b). Previous studies have shown that cancer cells migrate more efficiently through softer hydrogel matrices with lower mechanical strengths that is why we tested two different hydrogel dilutions. The images show that the invasion in both dilutions is very similar in VitroGel RGD. This indicates that both dilutions are adequate to observe the U87-MG glioblastoma invasion. The strength of the 1:5 and 1:10 hydrogel has been calculated to be less than 100 Pa. Reducing the dilution to 1:3 (≈200 Pa) or 1:1 (≈ 2000 Pa) will likely influence the invasion patterns of the cell, the cell morphology, and the cell-cell interactions. The cells might encounter more physical stress as they moved through the matrix. The mechanical stress can also affect the way the cells communicate with other cells and their environment, which might lead to different gene expression patterns.
On the other hand, in the 1:10 diluted VitroGel 3D, more cells are at the bottom of the insert compared to the 1:5 diluted Vitrogel 3D. In this case, the cells within the hydrogel have poor cell-matrix interactions since the hydrogel is unmodified however, invasion still occurs to an extent because of the lower mechanical strength of the 1:10 diluted hydrogel. It is very helpful for researchers to have a system such as VitroGel, which allows for the evaluation of multiple biophysical properties of the tumor microenvironment.
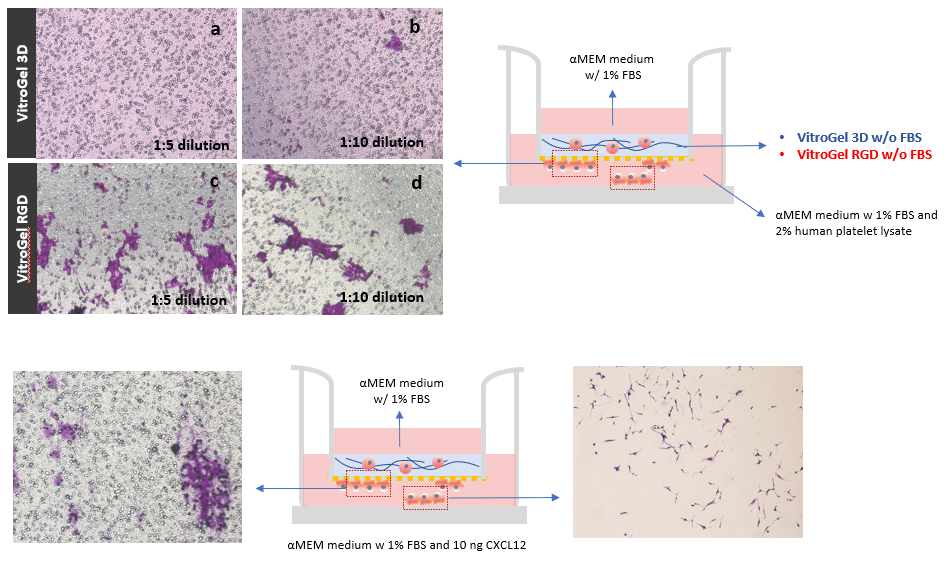
Figure 2: The cells in VitroGel 3D (a & b) remained within the hydrogel matrix while the cells cultured in VitroGel RGD (c & d) interacted with the matrix to move to the bottom of the Transwell insert. The images of the bottom of the Transwell were obtained on day 2. Induction of U87-MG glioblastoma cell invasion through a VitroGel RGD hydrogel by chemokine CXCL12. VitroGel RGD was used at a 1:5 dilution ratio. The medium outside of the insert well contained 1% FBS and 10 ng CXCL12. Cells at the bottom of the well plate (e) and at the bottom of the well plate were imaged on day 7.
Glioblastoma invasion induced by chemokine
Next, cells were exposed to CXCL12 to examine the induction of invasive behavior. To test this, U87-MG cells were culture on an RGD modified hydrogel (1:5 dilution) on a Transwell insert; CXCL12 was present in the medium outside the Transwell insert to act as an attractive cue. CXCL12 was found to induce 3D invasion of U87-MG glioblastoma cells through a hydrogel matrix modified with RGD (fig.2 e & f). Specifically, larger cell clusters were observed at the bottom of the Transwell insert in cultures treated with CXCL12 (fig. 2e) than without CXCL12 (fig. 2c). Some of the cells attached to the bottom of the 24 well plate, outside the Transwell insert (fig 2f). These results illustrate that VitroGel RGD is an effective system for testing the
effects of pro-tumor factors on cancer cell invasion and proliferation.
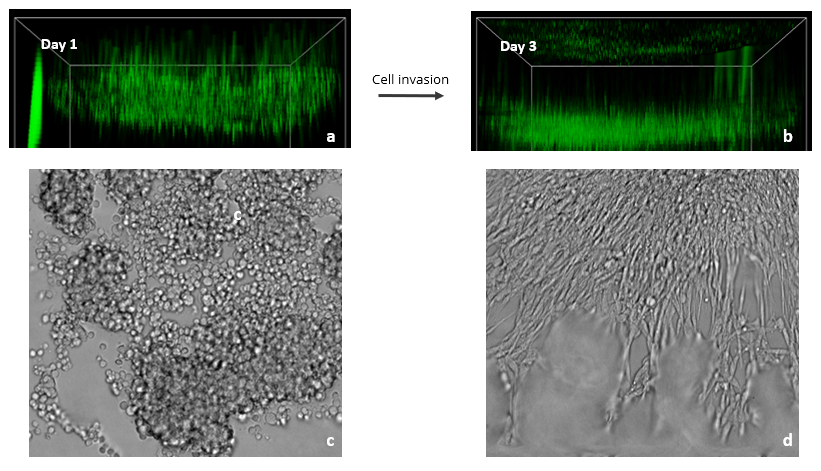
Figure 3: Images showing the spread of Glioblastoma U87-MG cells in a VitroGel RGD hydrogel matrix. The XYZ stack images (a – day 1& b – day 3) show green-labeled U87-MG cells (calcein AM) at different layers migrating toward an FBS gradient. XYZ projection images show epithelial projections from cell clusters (c – day 1& d – day 3).
Glioblastoma invasion induced by chemokine
Since the FBS induced Transwell invasion showed that U87-MG invasion is more prominent in the RGD modified hydrogel, we tested 2D coating hydrogel invasion in a 96 well plate using VitroGel RGD. Z-stack images in figure 4 show cell spread in the Z-plane on day 1 and day 3. Glioblastoma cells readily attached to the hydrogel surface and subsequently penetrated the matrix towards the FBS gradient (Fig. 3). Specifically, RGD promoted cell-matrix interactions and FBS acted as a chemoattractant for U87-MG cells. XYZ projection images showed several cell clumps on day 1 that, by day 3, aggregated together and spread out. These cell clusters exhibited characteristic epithelial morphologies, likely as a result of the presence of the RGD binding ligand in the hydrogel. Cell clustering is indicative of strong cell-cell interactions, and the migration we observed demonstrates the ability of FBS to promote cell-matrix binding. Overall, this experiment shows the impressive ability of VitroGel RGD to provide a platform for studying and imaging 3D cell migration and invasion.
Discussion
The data presented here illustrate the value of the VitroGel system for evaluating the intrinsic and extrinsic factors that govern cancer cell proliferation, migration, and invasion. Previously, VitroGel has been shown to support tumor spheroid growth and proliferation16–19. Moreover, our data reveal that RGD-mediated cell-cell and cell-matrix interactions play a significant role in the cell invasion. We found that both Transwell Inserts and 96-well plates provide a useful platform for studying 3D migration and invasion in VitroGel hydrogels.
The full understanding of the influence of the ECM on cancer growth and development requires a system that can recapitulate ECM composition while remaining user friendly20. VitroGel offers a variety of adhesive ligands that provide specialized bioactivity for tumor cell adhesion, growth, proliferation, migration, and invasion. The system allows researchers the ability to combine, alter, and control individual peptide concentrations in order to perform comprehensive testing of the optimal condition required for a given cell type. In this way, researchers can evaluate cancer cell behavior in physiologically relevant contexts while maintaining consistency and reproducibility. A suitable system for studying ECM-mediated effects on cancer development and progression must also be easily adjustable, as multiple studies have shown the effects of differential ECM biophysical properties and composition on various cancer cell types20,21. Therefore, to produce clinically relevant disease models for cancer research and drug development, researchers must tune the biochemical and biophysical factors of the extracellular milieu to realistically represent in vivo cell-cell and cell-matrix interactions. Since VitroGel can be modulated into a wide range of stiffness, researchers can investigate the effects of mechanical stress on tumor cell organization and spreading. Understanding the mechanisms that govern tumor cell-matrix interactions and adaptations to different surface tensions and mechanical strengths is critically important for the development of anti-proliferative and anti-metastatic pharmacological treatments22,23.
While VitroGel provides an excellent platform for studying cancer cells, it may also be useful in studies of developmental biology, immunology, and regenerative medicine. As such, invasion and migration assays are useful for investigating important molecular hubs and signaling in disease formation and progression. Moreover, the isolation of different conditional environments can help ferret out the importance of different factors. Having consistent, defined conditions is critically important to ensure no extraneous cell-matrix interactions affect the reporting of results. Importantly, the use of an animal-derived matrix (such as Matrigel) can result in such confounding factors. Therefore, the use of a xeno-free system presents researchers with a more reliable, and adjustable, platform to accommodate different experimental designs. The studies presented here provide evidence that the VitroGel hydrogel is a viable solution to cancer and regenerative medicine studies. Furthermore, the tunable and functional hydrogel system can be manipulated to study many different factors including mechanical strength, effects of binding ligands on cell invasion, and mobility.
Reference
- Eisemann, T., Costa, B., Strelau, J., Mittelbronn, M., Angel, P., and Peterziel, H. (2018). An advanced glioma cell invasion assay based on organotypic brain slice cultures. BMC Cancer.
- Cong, S., Luo, H., Li, X., Wang, F., Hua, Y., Zhang, L., Zhang, Z., Li, N., and Hou, L. (2019). Isatin inhibits SH-SY5Y neuroblastoma cell invasion and metastasis through PTEN signaling. Int. J. Clin. Exp. Pathol.
- Stock, K., Estrada, M.F., Vidic, S., Gjerde, K., Rudisch, A., Santo, V.E., Barbier, M., Blom, S., Arundkar, S.C., Selvam, I., et al. (2016). Capturing tumor complexity in vitro: Comparative analysis of 2D and 3D tumor models for drug discovery. Sci. Rep.
- Wu, M., and Melody, A.S. (2014). Modeling tumor microenvironments in Vitro. J. Biomech. Eng.
- Caliari, S.R., and Burdick, J.A. (2016). A practical guide to hydrogels for cell culture. Nat. Methods.
- Anton, D., Burckel, H., Josset, E., and Noel, G. (2015). Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci.
- Kim, Y., and Kumar, S. (2014). CD44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motility. Mol. Cancer Res.
- Ananthanarayanan, B., Kim, Y., and Kumar, S. (2011). Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials.
- Loessner, D., Stok, K.S., Lutolf, M.P., Hutmacher, D.W., Clements, J.A., and Rizzi, S.C. (2010). Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials.
- Artym, V. V., Swatkoski, S., Matsumoto, K., Campbell, C.B., Petrie, R.J., Dimitriadis, E.K., Li, X., Mueller, S.C., Bugge, T.H., Gucek, M., et al. (2015). Dense fibrillar collagen is a potent inducer of invadopodia via a specific signaling network. J. Cell Biol.
- Pathak, A., and Kumar, S. (2011). Biophysical regulation of tumor cell invasion: Moving beyond matrix stiffness. Integr. Biol.
- Liu, P., Long, P., Huang, Y., Sun, F., and Wang, Z. (2016). CXCL12/CXCR4 axis induces proliferation and invasion in human endometrial cancer. Am. J. Transl. Res.
- Witsch, E., Sela, M., and Yarden, Y. (2010). Roles for Growth Factors in Cancer Progression. Physiology.
- Lee, K.S., Jung, H.S., Moon, S., and Choe, G. (2017). Selection of a highly invasive cell population from a glioblastoma cell line and analysis of invasion-associated factors. Int. J. Clin. Exp. Pathol.
- Righetti, A., Giulietti, M., Šabanović, B., Occhipinti, G., Principato, G., and Piva, F. (2019). CXCL12 and Its Isoforms: Different Roles in Pancreatic Cancer? J. Oncol.
- Kim, E.J., Yang, C., Lee, J., Youm, H.W., Lee, J.R., Suh, C.S., and Kim, S.H. (2020). The new biocompatible material for mouse ovarian follicle development in three-dimensional in vitro culture systems. Theriogenology.
- Thanindratarn, P., Li, X., Dean, D.C., Nelson, S.D., Hornicek, F.J., and Duan, Z. (2019). Establishment and Characterization of a Recurrent Osteosarcoma Cell Line: OSA 1777. J. Orthop. Res.
- Shamloo, B., Kumar, N., Owen, R.H., Reemmer, J., Ost, J., Serene Perkins, R., and Shen, H.Y. (2019). Dysregulation of adenosine kinase isoforms in breast cancer. Oncotarget.
- Huang, J. (2019). 3D Cell Culture On VitroGel System. Cytol. Tissue Biol.
- Melissaridou, S., Wiechec, E., Magan, M., Jain, M.V., Chung, M.K., Farnebo, L., and Roberg, K. (2019). The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int.
- Riedl, A., Schlederer, M., Pudelko, K., Stadler, M., Walter, S., Unterleuthner, D., Unger, C., Kramer, N., Hengstschläger, M., Kenner, L., et al. (2017). Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J. Cell Sci.
- Yuan, H., Xing, K., and Hsu, H.Y. (2018). Trinity of three-dimensional (3D) scaffold, vibration, and 3D printing on cell culture application: A systematic review and indicating future direction. Bioengineering.
- Nagai, Y., Yokoi, H., Kaihara, K., and Naruse, K. (2012). The mechanical stimulation of cells in 3D culture within a self-assembling peptide hydrogel. Biomaterials.