Application Notes
Long Term 3D Tumor Spheroid Culture in VitroGel® Hydrogel Matrix
Application Note
Nana-Fatima Haruna, Bhavya Shah, John Huang
TheWell Bioscience, North Brunswick, NJ 08902
Introduction
Cancer is a very meticulous disease that involves heterogeneous solid tumors subjected to complex extracellular conditions. These conditions, such as hypoxia and extracellular compositions, contribute to the pathogenesis of the disease. The functional activity and gene expression patterns of the tumor cells are different from the normal tissue. This factor makes the disease more difficult to understand, control, and treat1-3. Consequently, researchers have begun to switch their in vitro culture methods from 2D monolayer systems to 3D spheroid models to help the cancer cells regain intrinsic properties and depict a more realistic cellular response to drugs. The intrinsic properties at play here include cell-cell and cell-matrix interaction (complex stroma-cancer interactions). It is also important to form a 3D tumor model for long-term culture, in which the large tumor spheroids (e.g. > 200 μm) can generate an external proliferating zone for metastasis and other aspects of the disease’s pathophysiology 3. Moreover, the large tumor size will allow the creation of an oxygen and nutrient gradient within the tumor, which represents a key feature of in vivo tumors 3-5. These kinds of models will allow for the generation of physiologically relevant data pertaining to oncogenesis, which will ultimately lead to the development of more reliable therapeutic interventions to combat the disease.
The traditional animal-based extracellular matrixes (ECM) introduce some experimental obscurities because of the presence of unknown biochemical components 4. These matrixes are also obtained from non-human specimens, generating inaccuracy in the study of human diseases such as pancreatic and colon cancer 4, 5, 6. The animal origin-free VitroGel hydrogel provides a robust 3D cell culture platform allowing for the study and generation of clinically relevant data in these diseases. VitroGel® Hydrogel Matrix (VHM, Cat# VHM01) is an optimized formulation of multi-functional ligands to stimulate cell-cell and cell-matrix interactions to support a wide range of cell types for different applications. The system provides a more robust range of properties to better understand the complicated relationship between cells and matrix components.
In this study, we established the in vitro 3D tumor models of human pancreatic cancer cells (PANC-1) and colon cancer cell lines (HCT116) in VitroGel Hydrogel Matrix to support long-term culture study. In real cases, tumors may grow for months or years before being diagnosed. This results in the formation of tumor spheroids with a necrotic core. The necrotic core is impermeable to nutrients, oxygen, and even drugs which eventually leads to chemoresistance. The 3D tumor models established in this study can serve as a drug testing model for solid tumors. Also, we compared the cell growth in VitroGel RGD (V-RGD, Cat# TWG003), a single functional ligand modified hydrogel, and VitroGel Hydrogel Matrix, the multi-functional ligand modified hydrogel to see how the synergistic effect of the ECM binding ligand effect the tumor formation. The goal is to build sophisticated cancer models that reflect the intricacies of the cellular and molecular mechanisms of the disease, which can pave the way for a better understanding of carcinogenesis and developing physiological-relevant data. Promising results will open doors for several quantitative, qualitative, and downstream analyses providing a new platform for cancer research and treatment options.
Materials and Methods
Cell Maintenance
HCT116 and PANC-1 cells were maintained with McCoy’s 5a and DMEM, respectively. The media were supplemented with 10% FBS, 1x pen-strep, and 1x amphotericin. Cells were passaged when the cultures reached ~80% confluence.
3D Culture
About 1×106 cells/mL were resuspended in a medium containing 50% FBS for the VitroGel RGD and 30% FBS for VitroGel Hydrogel Matrix. The RGD gel used to compared with VitroGel Hydrogel Matrix was prepared using the high concentration VitroGel RGD diluted with VitroGel Dilution Solution Type 1 (Cat# MS01-100) at 1:3 ratio (v/v). The diluted hydrogel solution was then mixed with cell suspension at 4:1 v/v ratio. (The details of 3D cell culture protocol for VitroGel RGD can be found at https://www.thewellbio.com/wp-content/uploads/2019/12/VitroGel-Handbook.pdf).
For 3D cell culture with the ready-to-use VitroGel Hydrogel Matrix, the hydrogel solution was directly mixed with cell suspension at 2:1 (v/v) mixing ratio. (The detailed protocol for 3D culture for VitroGel Hydrogel Matrix can be found at https://www.thewellbio.com/wp-content/uploads/2020/05/VitroGel-Hydrogel-Matrix-TDS.pdf)
50 µl of the hydrogel mix was then transferred to a 96 well plate. The gel was allowed to stabilize at room temperature for 15 minutes before adding 50 µl cover medium on the top of the hydrogel.
Fluorescence Microscopy
Cells were fixed and stained by using the Image-iT® Fixation/Permeabilization Kit, ActinGreen™ 488 ReadyProbes® Reagent, and NucBlue® Fixed Cell ReadyProbes™ Reagent (ThermoFisher) according to VitroGel fluorescent staining protocol (https://www.thewellbio.com/wp-content/uploads/2020/08/Protocol_Cell-Fluorescent-Staining-in-Gel-for-Nuclei-and-Actin-Filament.pdf.) The Z-stack projection images were obtained by using ImageExpress system from Molecular Devices.
Proliferation Assay
Proliferation was measured using Cell Counting kit-8 (CCK-8). Cells were prepared with VitroGel Hydrogel Matrix and seeded at a density of 2,000 cells/well in a 96 well plate for 3D cell culture. 10 µl CCK8 was added to each well that contained 50 µl gel with 50 µl cover medium (about 350 cells/well). Cells were incubated at a 37 °C incubator for 2 hours before reading the absorbance at 450 nm by using a microplate reader.
Live/Dead Assay
Cyto3D™ Live-Dead Assay Kit (Cat# BM01) was used to visually track the proliferation of HCT116 and PANC-1 cells. 2 µl of the Cyto3D solution was added to each well that contained 50 µl gel with 50 µl cover medium. The cells were then incubated at a 37 °C incubator for 15 minutes before imaged by imaging.
Results
Comparison of VitroGel Hydrogel Matrix and VitroGel RGD on 3D cell structure formation
PANC- 1 cells were 3D cultured in both VitroGel Hydrogel Matrix and VitroGel RGD a for 7 days. Figure 1 a & c show the single-cell suspension in both hydrogels at day 1. After 7 days of culture, 3D cell spheroids formed in both hydrogel (Figure 1 b& d). However, the 3D cell structures in VitroGel Hydrogel Matrix hydrogel were larger with more rough edges than the cells in the VitroGel RGD. Although the integrin-binding ligand RGD performs an important role in supporting the cell-matrix interaction for the pancreatic spheroid formation and cell proliferation, 6 the growth of PANC-1 spheroids in VitroGel Hydrogel Matrix indicated the synergistic effects of multiple functional ligands in VitroGel Hydrogel Matrix might increase the bioactivity and enhanced cell growth, cell-cell, and cell-matrix interactions, creating a more realistic 3D model for solid tumor study.
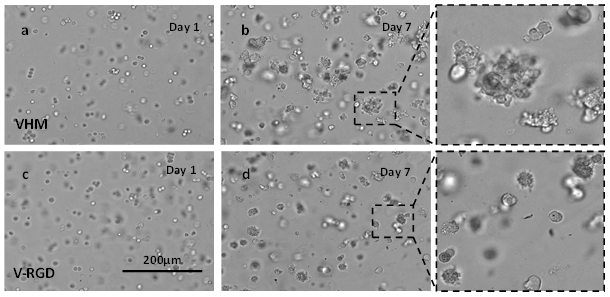
Figure 1: 3D culture of PANC-1 cells in VitroGel RGD (V-RGD) and VitroGel Hydrogel Matrix (VHM)
a & b PANC-1 cells in VHM on day 1 and day 7; c & d PANC-1 cells in the RGD on day 1 and day 7; the enlarged images show the 3D tumor spheroids in both hydrogels after 7-day culture.
Proliferation assay and cell Size as a function of time
The proliferation rate of PANC-1 VitroGel Hydrogel Matrix was studied using CCK-8. Figure 2a indicates a sigmoidal increase in the cell number over the 3 weeks culture period. The cell number increased from 2,000 cells/well to over 1.5 x 105 cells/well in 3 weeks. We picked a low cell seeding density (2,000 cells/well) for the proliferation assay to facilitate the long-term 3D culture without overgrowth (usually, the recommended concentration of cells suspension for VitroGel 3D cell culture is 0.5-2 x 106 cells/ml, which makes 0.8-3.3 x 104 cells/well for final density). Therefore, the cell growth rate was slow at the first few days until the cell density reached around 4 x 104 cells/well (Figure 2a), then the cells show exponential growth in 3D. The size of the PANC-1 spheroids also increased as a function of time. Figure 2b shows the increase of spheroid diameters from 20 µm to 210 µm in 3 weeks.
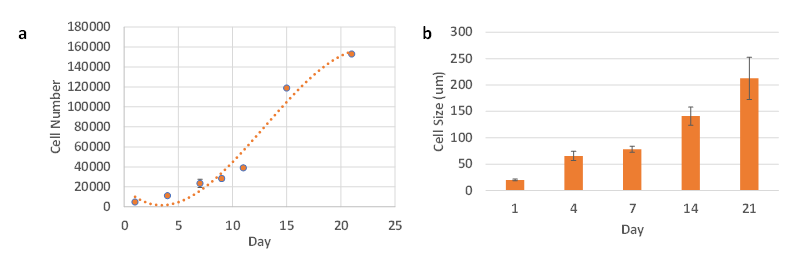
Figure 2. Proliferation assay and cell size of PANC-1 cells in VitroGel Hydrogel Matrix
Live/Dead assay
After long-term culture, the cell viability was evaluated by Cyto3D Live-Dead cell imaging assay. Figure 3 shows that PANC-1 spheroids were healthy with a minimal number of dead cells at day 14. In VitroGel Hydrogel Matrix, the cells were able to grow over 100 µm in diameter without significant cell death or necrosis. It is important to note that the soft hydrogel matrix’s flexibility allows the expansion of cell spheres while keeping them embedded during the entire experiment. The high cell viability of the spheroids also indicates the ability of oxygen and nutrients to penetrate the matrix and support larger cell structures. The results suggest VitroGel Hydrogel Matrix can be used for pharmacology and toxicology studies, drug screening, and other long-term studies.
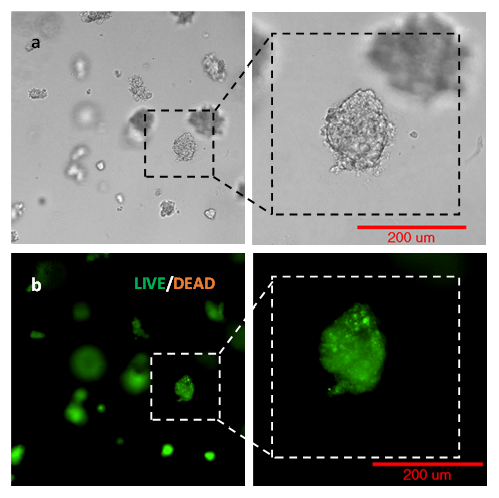
Figure 3. Live Dead imaging of PANC-1 spheroids using Cyto3D Live-Dead assay.
HCT116
Colon cancer cell line (HCT116) was also culture in VitroGel Hydrogel Matrix hydrogel for 3D cell structure formation. Figure 4 shows the growth of HCT116 cells from single cells (Figure 4a at day 1) to 3D spheroid structure (Figure 4b at day 7) in VitroGel Hydrogel Matrix. Similar to the PANC-1 3D spheroid structure, the enlarged image of day 7 showed a rough edge of the spheroid morphology, indicating a strong cell-matrix interaction that might enhance the cell bioactivities and proliferation in 3D.
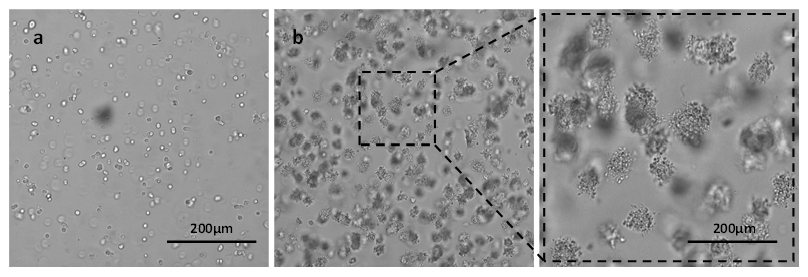
Figure 4. 3D culture of HCT116 in VitroGel Hydrogel Matrix at day 1 (a) and day 7 (b)
Proliferation assay and cell size as a function of time
Figure 5a shows the increase of cell number of HCT116 cells cultured in VitroGel Hydrogel Matrix for 3 weeks. Similar to PANC-1 cells, the cell number of 3D culture HCT116 cells increased from 2,000 cells/well to around 1.3 x 105 cells/well in 3 weeks. Because of the low cell seeding density (2,000 cells/well), the cell growth rate was slow at the first few days until the cell density reached around 2 x 104 cells/well (Figure 5a), then the cells show exponential growth in 3D. The size of the HCT116 spheroids also increased as a function of time. Figure 2b shows the increase of spheroid diameters from less than 20 µm to over 150 µm in 3 weeks.
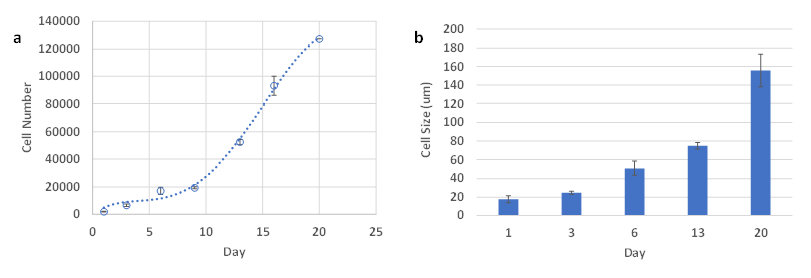 Figure 5. Proliferation assay and cell size of HCT116 cells in VitroGel Hydrogel Matrix. n= 2-3. Standard deviation was plotted as error bars. A. Proliferation assay of HCT116 over a period of 3 weeks B. Cell size of HCT116 over a period of 3 weeks
Figure 5. Proliferation assay and cell size of HCT116 cells in VitroGel Hydrogel Matrix. n= 2-3. Standard deviation was plotted as error bars. A. Proliferation assay of HCT116 over a period of 3 weeks B. Cell size of HCT116 over a period of 3 weeks
Live/Dead assay
The cell viability of HCT116 3D spheroid was also evaluated by Cyto3D Live-Dead cell imaging assay. Figure 6 shows the HCT116 3D spheroids with high cell viability. The cells encapsulated in the hydrogel matrix were able to grow into a 150 µm 3D structure without significant cell death or necrosis in VitroGel Hydrogel Matrix.
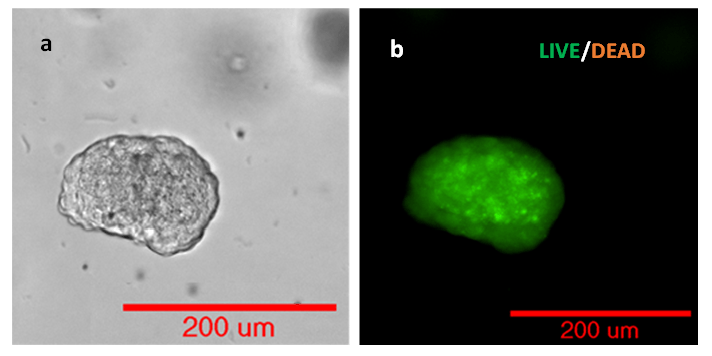
Figure 6. Live Dead imaging of HCT116 using Cyto3D Live-Dead Assay.
Discussion
Tumor heterogeneity is a major cause of resistance and poor health outcome in a cancer diagnosis 7. A better understanding of the distinct morphological and phenotypic profiles of cancer can direct cancer research towards a more efficient way to circumvent chemotherapy resistance and produce therapeutics 2. Only 10% of the screened drugs move on successfully through clinical trials 9. The low rate is due to the failure of cells in 2D culture to interact with their surroundings, making the process of drug discovery ineffective 9,10. Therefore, it is imperative to develop a system that can mimic the in vivo environment and produce more predictable results.
Cell culture in VitroGel Hydrogel Matrix can serve as a model system used to study tumor morphology and mechanisms that lead to tumor resistance. The culture of cancer cells in VitroGel Hydrogel Matrix will help us study the oxygen and nutrient gradient from the necrotic core to the proliferative zone8. The above results provide evidence that VitroGel Hydrogel Matrix facilitates proliferation and supports the high cell viability of PANC-1 and HCT116 in 3D cell culture. The study shows the multiple functional xeno-free hydrogel system can enable a comprehensive 3D cell model and provide a better understanding of cancer pathophysiology.
The multifunctionality of this platform also supports the development of fibroblast, epithelial, complex cellular networks, and increased cell-matrix and cell-cell interactions. This paves the way for heterotypic co-culture studies, increasing the complexity of the in vitro system. The mechanisms of interactions between cancer cells and immune cells can also be explored in order to identify drug targets and improve the outcome of cancer immunotherapies10-12. Additionally, the xeno-free system eliminates the undesired animal elements and generates clinically relevant, precise, and reproducible data.
Another application of the system is the culturing of patient-derived cells for personalized medicine. Personalized medicine practices today identify genetic mutations in disease models and matching those mutations to therapeutics13. However, the relationship between those mutations and the drugs has not yet been completely explored. The VitroGel system can be used to culture patient-derived cells to perform pharmacological screening and investigate the drug resistance on in vitro 3D cell models14-16. Consequently, individual patient care can be impacted, matching therapies to specific patients within weeks of the biopsy.
In essence, the need for better therapeutics in cancer warrants a model system that is more representative of the in vivo environment. VitroGel system allows for robust use with different cell types and in varying conditions.
Reference
- ZEEBERG, KATRINE, et al. “Assessment of Different 3D Culture Systems to Study Tumor Phenotype and Chemosensitivity in Pancreatic Ductal Adenocarcinoma.” International Journal of Oncology, vol. 49, no. 1, 10 May 2016, pp. 243–252, www.spandidos-publications.com/10.3892/ijo.2016.3513, 10.3892/ijo.2016.3513. Accessed 12 Oct. 2020.
- Lv, Donglai, et al. “Three‑dimensional Cell Culture: A Powerful Tool in Tumor Research and Drug Discovery (Review).” Oncology Letters, vol. 14, no. 6, 3 Oct. 2017, www.spandidos-publications.com/ol/14/6/6999, 10.3892/ol.2017.7134. Accessed 2 Mar. 2020.
- Cavo, Marta, et al. “A Synergic Approach to Enhance Long-Term Culture and Manipulation of MiaPaCa-2 Pancreatic Cancer Spheroids.” Scientific Reports, vol. 10, no. 1, 23 June 2020, p. 10192, www.nature.com/articles/s41598-020-66908-8#Sec1, 10.1038/s41598-020-66908-8.
- Jabbari, Esmaiel. “Challenges for Natural Hydrogels in Tissue Engineering.” Gels, vol. 5, no. 2, 29 May 2019, p. 30, www.ncbi.nlm.nih.gov/pmc/articles/PMC6631000/, 10.3390/gels5020030. Accessed 25 Aug. 2019.
- Zhu, Xiaolu, and Xianting Ding. “Study on a 3D Hydrogel-Based Culture Model for Characterizing Growth of Fibroblasts under Viral Infection and Drug Treatment.” SLAS DISCOVERY: Advancing the Science of Drug Discovery, vol. 22, no. 5, 24 Mar. 2017, pp. 626–634, journals.sagepub.com/doi/full/10.1177/2472555217701247, 10.1177/2472555217701247. Accessed 12 Oct. 2020.
- Zustiak, Silviya P., et al. “Influence of Cell-Adhesive Peptide Ligands on Poly(Ethylene Glycol) Hydrogel Physical, Mechanical and Transport Properties.” Acta Biomaterialia, vol. 6, no. 9, 1 Sept. 2010, pp. 3404–3414, www.ncbi.nlm.nih.gov/pmc/articles/PMC2910244/, 10.1016/j.actbio.2010.03.040.
- Cui, X., et al. “Advances in Multicellular Spheroids Formation.” Journal of The Royal Society Interface, vol. 14, no. 127, Feb. 2017, p. 20160877, www.ncbi.nlm.nih.gov/pmc/articles/PMC5332573/, 10.1098/rsif.2016.0877.
- Dagogo-Jack, Ibiayi, and Alice T. Shaw. “Tumour Heterogeneity and Resistance to Cancer Therapies.” Nature Reviews Clinical Oncology, vol. 15, no. 2, 8 Nov. 2017, pp. 81–94, www.nature.com/articles/nrclinonc.2017.166, 10.1038/nrclinonc.2017.166. Accessed 4 Apr. 2019.
- Kapałczyńska, Marta, et al. “2D and 3D Cell Cultures – a Comparison of Different Types of Cancer Cell Cultures.” Archives of Medical Science, vol. 14(4), no. 910, 2016, www.ncbi.nlm.nih.gov/pmc/articles/PMC6040128/, 10.5114/aoms.2016.63743. Accessed 9 Dec. 2019.
- Edmondson, Rasheena, et al. “Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors.” ASSAY and Drug Development Technologies, vol. 12, no. 4, May 2014, pp. 207–218, www.ncbi.nlm.nih.gov/pmc/articles/PMC4026212/, 10.1089/adt.2014.573.
- Courau, Tristan, et al. “Cocultures of Human Colorectal Tumor Spheroids with Immune Cells Reveal the Therapeutic Potential of MICA/B and NKG2A Targeting for Cancer Treatment.” Journal for ImmunoTherapy of Cancer, vol. 7, no. 1, 14 Mar. 2019, 10.1186/s40425-019-0553-9. Accessed 16 Oct. 2020.
- Norberg, K. J., et al. “A Novel Pancreatic Tumour and Stellate Cell 3D Co-Culture Spheroid Model.” BMC Cancer, vol. 20, 27 May 2020, www.ncbi.nlm.nih.gov/pmc/articles/PMC7251727/, 10.1186/s12885-020-06867-5. Accessed 12 Oct. 2020.
- Zoetemelk, Marloes, et al. “Short-Term 3D Culture Systems of Various Complexity for Treatment Optimization of Colorectal Carcinoma.” Scientific Reports, vol. 9, 8 May 2019, www.ncbi.nlm.nih.gov/pmc/articles/PMC6506470/, 10.1038/s41598-019-42836-0. Accessed 12 Oct. 2020.
- Kodack, David P., et al. “Primary Patient-Derived Cancer Cells and Their Potential for Personalized Cancer Patient Care.” Cell Reports, vol. 21, no. 11, Dec. 2017, pp. 3298–3309, www.ncbi.nlm.nih.gov/pmc/articles/PMC5745232/, 10.1016/j.celrep.2017.11.051. Accessed 22 Sept. 2019.
- Badodi, Sara, et al. “Establishment and Culture of Patient-Derived Primary Medulloblastoma Cell Lines.” Methods in Molecular Biology (Clifton, N.J.), vol. 1869, 2019, pp. 23–36, pubmed.ncbi.nlm.nih.gov/30324511/, 10.1007/978-1-4939-8805-1_3. Accessed 16 Oct. 2020.
- Stringer, Brett W., et al. “A Reference Collection of Patient-Derived Cell Line and Xenograft Models of Proneural, Classical and Mesenchymal Glioblastoma.” Scientific Reports, vol. 9, no. 1, 20 Mar. 2019, p. 4902, www.nature.com/articles/s41598-019-41277-z, 10.1038/s41598-019-41277-z. Accessed 16 Oct. 2020.