Research Highlights
Establishing the First Co-Culture Model of Human Gastric Organoids and Dendritic Cells Using the Xeno-free VitroGel ORGANOID Hydrogel
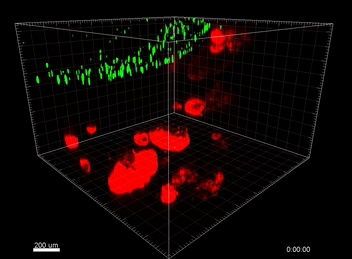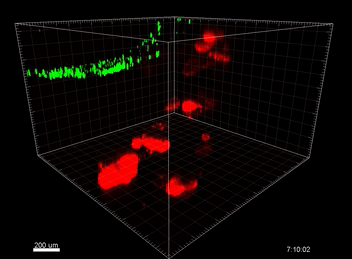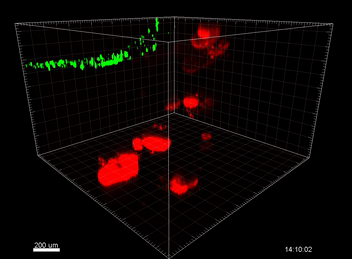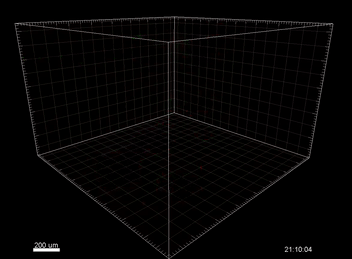
VitroGel® ORGANOID
Matrigel
Institutions:
Department of Microbiology and Cell Biology, Montana State University, Bozeman, MT, United States
Team:
Michelle D. Chergne, Barkan Sidar, T. Andrew Sebrell, Humberto S. Sanchez, Kody Heaton, Francis J. Kassama, Mandi M. Roe, Andrew B. Gentry, Connie B. Chang, Seth T. Walk, Mark Jutila, James N. Wilking, and Diane Bimczok
Application:
A Synthetic Hydrogel, VitroGel® ORGANOID-3, Improves Immune Cell-Epithelial Interactions in a Tissue Chip Co-Culture Model of Human Gastric Organoids and Dendritic Cells
Biological Model:
Co-Culture of human gastric organoids and dendritic cells using the VitroGel® ORGANOID-3 system
Hydrogel:
VitroGel® ORGANOID
Understanding the immunosurveillance of the gastrointestinal epithelium by mononuclear phagocytes (MNPs) is essential for proper gut health. Diane Bimczok’s research group from Montana State University focuses on studying the complex interplay between the human gastrointestinal epithelium and MNPs such as dendritic cells (DCs), which is critical for the maintenance of gastrointestinal (GI) homeostasis. However, the transwell chemotaxis assays and live confocal imaging revealed that the traditional organoid culture method with Matrigel prevented DC migration towards the organoids and the establishment of direct MNP-epithelial contacts. Although Matrigel is a commonly used material for organoid culture, it has a poorly defined composition, shows significant batch effect variability across different studies, and is associated with animal welfare concerns, thus hindering its use as a clinical application for organoid technologies in the future. 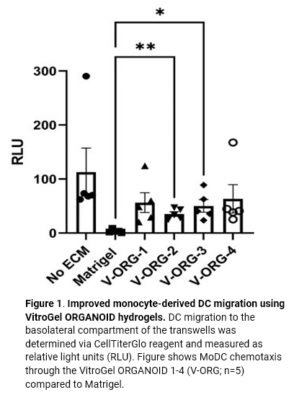 Because of the complexity of this animal-based matrix system, it is extremely difficult to adjust the properties of the Matrigel matrix to improve cell migrations. After adjusting the concentrations of Matrigel and mixing Matrigel with collagen at different ratios but without showing any improvement on cell migration, Bimczok’s team began working with the xeno-free VitroGel ORGANOID hydrogel system. By comparing with multiple conditions of Matrigel and Matrigel-collagen mixture, the team showed that the synthetic VitroGel ORGANOID-3 (V-ORG-3) significantly enhanced dendritic cell chemotaxis through the matrix and provided markedly better support for organoid growth and proliferation, suggesting it is superior as an ECM material to support co-culture systems (Figure 1). DCs migrated rapidly through the VitroGel ORGANOID matrix and reached organoids to establish co-culture integration. Likewise, by incorporating a tissue chip system known as the gut organoid flow chip (GOFlowChip) platform, Bimczok’s team established a micro-physiological organoid system, which is the first of its kind, to permit real-time imaging of MNP-epithelial interactions and other investigations of the complex interplay between gastrointestinal MNPs and epithelial cells.
Because of the complexity of this animal-based matrix system, it is extremely difficult to adjust the properties of the Matrigel matrix to improve cell migrations. After adjusting the concentrations of Matrigel and mixing Matrigel with collagen at different ratios but without showing any improvement on cell migration, Bimczok’s team began working with the xeno-free VitroGel ORGANOID hydrogel system. By comparing with multiple conditions of Matrigel and Matrigel-collagen mixture, the team showed that the synthetic VitroGel ORGANOID-3 (V-ORG-3) significantly enhanced dendritic cell chemotaxis through the matrix and provided markedly better support for organoid growth and proliferation, suggesting it is superior as an ECM material to support co-culture systems (Figure 1). DCs migrated rapidly through the VitroGel ORGANOID matrix and reached organoids to establish co-culture integration. Likewise, by incorporating a tissue chip system known as the gut organoid flow chip (GOFlowChip) platform, Bimczok’s team established a micro-physiological organoid system, which is the first of its kind, to permit real-time imaging of MNP-epithelial interactions and other investigations of the complex interplay between gastrointestinal MNPs and epithelial cells.
The state-of-the-art, xeno-free VitroGel hydrogel system closely mimics the natural extracellular environment and enables high-throughput capable processing to establish a variety of 3D models. The hydrogel is a ready-to-use tool with most in vitro application protocols taking only 20 minutes. Moreover, the VitroGel system has excellent flexibility for biophysical and bio-functional properties modifications. As the research discussed in the following text of this highlight demonstrates, it is highly biocompatible, supporting a variety of cell types on organoid co-culture systems.
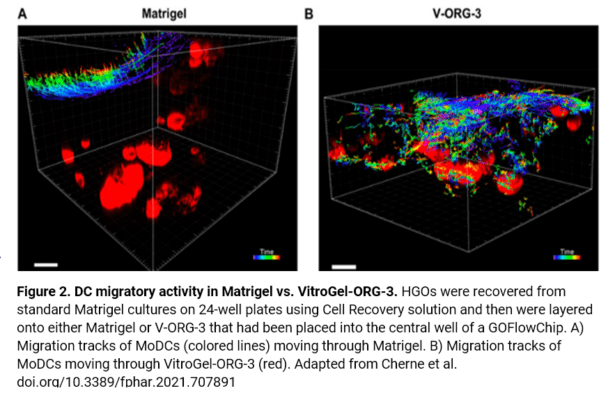
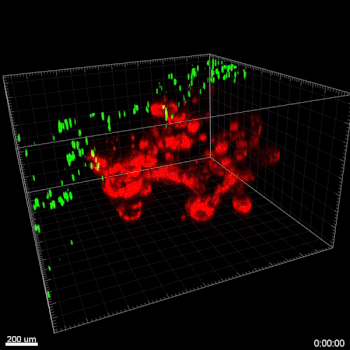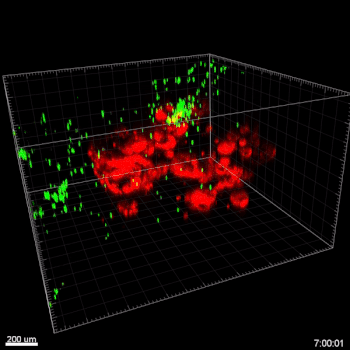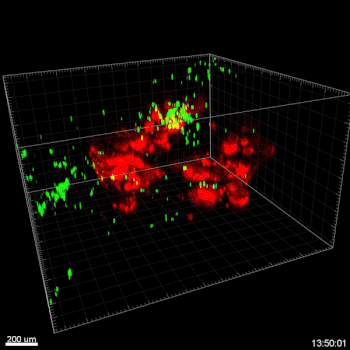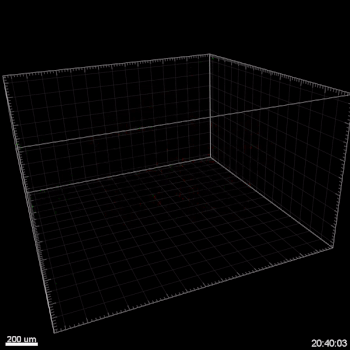
In this study, cell migration is a significant aspect of organoid culture models. Importantly, cell types such as DCs exhibit significantly improved cell migration when using the VitroGel® ORGANOID-3 culture system compared to Matrigel, as shown by live confocal microscopy images over a 20-hour period (Figure 2). Specifically, the MoDCs displayed minimal migratory activity towards organoids embedded deep in the ECM of the Matrigel (Figure 2A). In contrast, almost all MoDCs were able to penetrate ~500uM into the VitroGel® ORGANOID-3, as well as accumulate within proximity of the HGOs (Figure 2B). These data demonstrate that the VitroGel® organoid system confers markedly enhanced migratory activity for DCs and human gastric organoids (HGOs) compared to cells embedded within Matrigel.
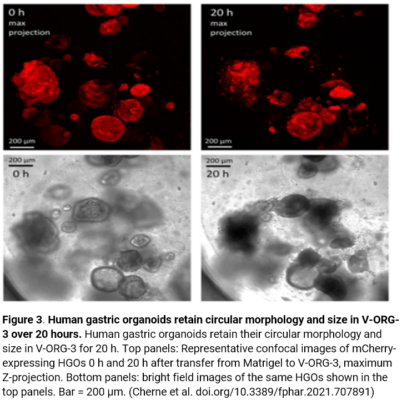 Likewise, HGOs were able to retain their circular morphology and size when embedded in the VitroGel® ORGANOID-3 co-culture system. Figure 3 depicts confocal images 20 hours after transfer from cells from Matrigel to the VitroGel® system (Figure 3). Ideal cell shape and size can clearly be observed over the 20-hour period, signaling that the V-ORG-3 hydrogel system is optimal for cell proliferation, can maintain organoid formation, and supports high cell viability during co-culture.
Likewise, HGOs were able to retain their circular morphology and size when embedded in the VitroGel® ORGANOID-3 co-culture system. Figure 3 depicts confocal images 20 hours after transfer from cells from Matrigel to the VitroGel® system (Figure 3). Ideal cell shape and size can clearly be observed over the 20-hour period, signaling that the V-ORG-3 hydrogel system is optimal for cell proliferation, can maintain organoid formation, and supports high cell viability during co-culture.
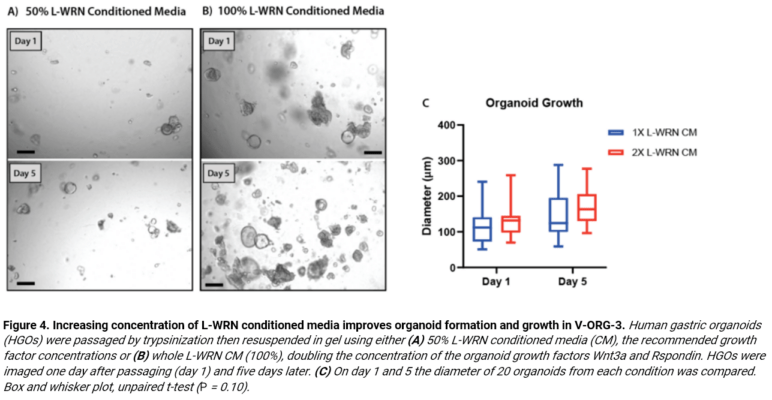
Finally, in comparisons of different supplements used within organoid cultures of the VitroGel® system, the scientists reveal that our synthetic hydrogel has immense flexibility, allowing a variety of hydrogel modifications or enrichments. This vast flexibility and ability to modify hydrogels based on what the scientist desires in Figure 4. Here, we demonstrate optimal organoid growth and proliferation of HGOs following passage via trypsinization and subsequent resuspension in the V-ORG-3 system that is supplemented various concentrations of the growth factor L-WRN in media over a 5-day period (Figure 4). Overall, these data display that the VitroGel® ORGANOID system is functionally modifiable and allows researchers to have better control over and optimize their co-culture systems by using defined supplements for enrichment.
The hydrogel is growth factor-free as well, meaning investigators have full control of the growth factors they may want to use to enrich the hydrogel system for organoid formation. We suggest mixing your critical growth factors/supplements at 3 times the normal concentration with the VitroGel ORGANOID hydrogel as the mixing ratio of the VitroGel ORGANOID hydrogel with the mixing medium (or cell suspension) is at a 2:1 ratio. For more information, see our VitroGel® ORGANOID (VHM04-1, VHM04-2, VHM04-3, VHM04-4, VHM04-K) user protocol.
In conclusion, the discovery from Diane Bimczok’s research group demonstrates the capability to establish the comprehensive 3D organoid co-culture system by using the xeno-free functional VitroGel® ORGANOID system. This study’s co-culture model of human gastric organoids and the monocyte-derived dendritic cells can easily be adapted for investigations in other areas with other cell types and tissue systems. The VitroGel® ORGANOID system overcomes a serious obstacle in the organoid co-culture field, which was the drawbacks of the animal-based matrix system. The system opens the door for comprehensive 3D models with organoid co-cultures, giving it a powerful tool for researchers to build tissue models for drug screening, motility, invasion assays, and tissue engineering.
Read the publication:
Related Products: