Application Notes
3D Spheroid Invasion Assay With the Xeno-free, Bio-Functional VitroGel® Hydrogel Matrix
Application Note
Nana-Fatima Haruna, John Huang
TheWell Bioscience, North Brunswick, NJ 08902
Introduction
- 50 µl of the cell suspension was added carefully into each well of a 96-well ultra-low attachment U-shaped plate
(S-BIO PrimeSurface 96U Plate – Cat# MS-9096UZ). - The plate was incubated at 37°C, 5% CO2 overnight, to obtain uniform glioblastoma spheroids for the invasion assay.
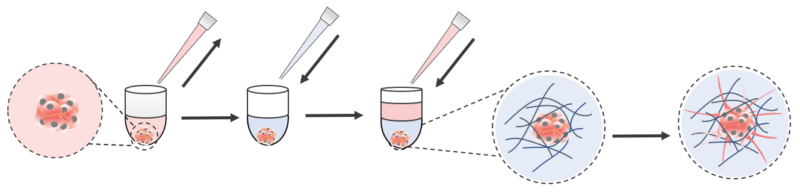
- The spheroids were carefully harvested before incorporating into the VitroGel hydrogels in two different invasion methods:
3D encapsulating invasion and 2D hydrogel coating invasion.
Hydrogel Preparation
The ready-to-use VitroGel® Hydrogel Matrix (Cat# VHM01) and the high-concentration VitroGel® 3D (Cat# TWG001) were selected in this experiment. VitroGel Hydrogel Matrix can be directly mixed with a cell culture medium for hydrogel formation. The VitroGel 3D was prepared at a 1:3 dilution with VitroGel Dilution Solution TYPE 1 (v/v) and 4:1 mixing (v/v) with the cell culture medium (αMEM + 50% FBS, please check VitroGel 3D user handbook for details).
2D Hydrogel Coating Invasion
(Using VitroGel Hydrogel Matrix as an example)
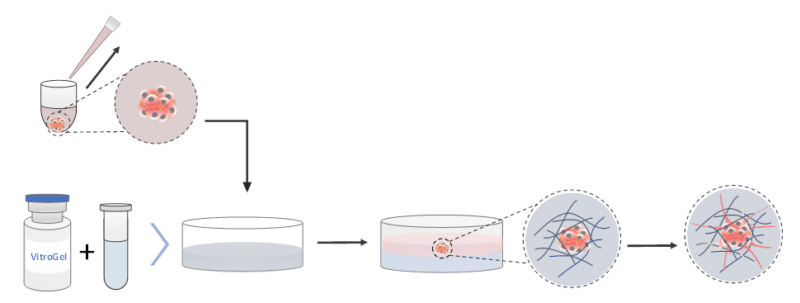
- The hydrogel solution was brought to room temperature or warmed up to 37°C.
- The hydrogel solution was mixed with the cell culture medium (αMEM + 30% FBS) at 2:1 (v/v) ratio in a microcentrifuge tube. (The mixing ratio was adjusted to 4:1 (v/v) when using VitroGel 3D.)
- 50 µl of the hydrogel-cell culture mixture was added to each well in a new flat-bottom 96-well plate.
- For smooth gel formation and stabilization, an incubation of 15–20 minutes at room temperature was allowed. During the formation process, do not disturb the hydrogel by tilting or shaking the well plate.
- The 50 µl of the medium and the formed spheroid from the U-shaped plate were carefully pipetted out. Care was taken not to disrupt the structure of the formed spheroid.
- The spheroid and medium were added to the top of the formed hydrogel in the flat-bottom plate. Care was taken not to introduce bubbles into the wells.
- The plate was immediately incubated at 37°C to complete the polymerization process.
- The cover medium was changed every 2–3 days by pipetting half of it out and replacing it with 50 µL of fresh medium. Care was taken not to disturb the hydrogel.
3D Encapsulation Invasion
(Using VitroGel® Hydrogel Matrix as an example)
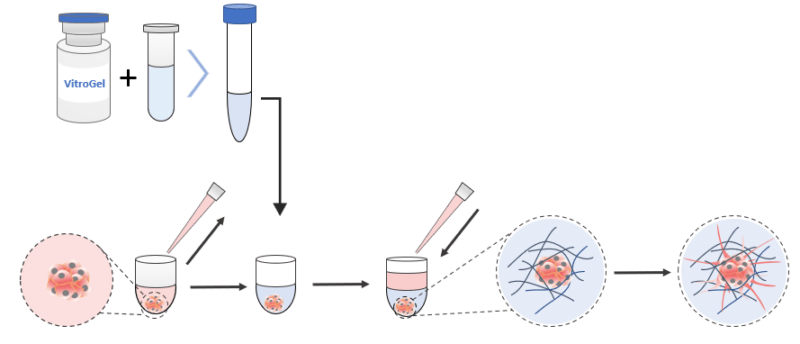
- The hydrogel solution was brought to room temperature or warmed up to 37°C.
- About 45 µL of the medium was carefully pipetted out of each well of the U-shaped plate. Care was taken not to disturb the structure of the formed spheroid nor to pipette it out.
- The hydrogel solution was mixed with the cell culture medium (αMEM + 30% FBS) at 2:1 (v/v) ratio in a microcentrifuge tube. (The mixing ratio was adjusted to 4:1 (v/v) when using VitroGel 3D.)
- 50 µl of the hydrogel mixture was immediately and carefully added to the U-shaped plate well with the spheroid.
- The mixture was pipetted twice to make sure the spheroid was correctly suspended in the hydrogel mixture. Care was taken not to pipette too hard to introduce bubbles nor to break apart the spheroid.
- The hydrogel was allowed to form for about 20 minutes at room temperature. The hydrogel was not disturbed by tilting or shaking the well plate during the formation process.
- The hydrogel was carefully covered with 50 µl cell culture medium (αMEM + 10% FBS) and incubated immediately at 37°C to complete the polymerization process.
- The cover medium was changed every 2-3 days by pipetting half of the cover medium out and replacing it with 50 µl of fresh medium. Care was taken not to disturb the hydrogel.
Measuring Cell Viability Using the Cyto3D® Live-Dead Assay Kit
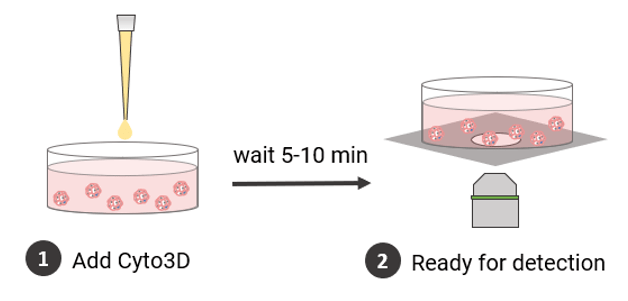
- The Cyto3D® Live-Dead Assay Kit (Cat# BM01) was brought to room temperature.
- The Cyto3D® reagent was added to the cover medium of the selected well (2 µL of Cyto3D® reagent to every 100 µL total volume in a well).
- The plate was incubated at 37°C for 5-10 minutes, after which the cells were ready for imaging to detect viability (following the product protocol for fluorescence microscope imaging details).
Calculating Cell Surface Area
The invasion process was tracked by imaging the cells over 7 days. The imaging was performed using an auto-microscope system, ImageXpress (Molecular Devices), and the image analysis was performed on MetaExpress High Content Image Acquisition and analysis software. We measured the cell area occupied by the growing U-87 MG spheroid and the metastatic invadopodia (the outgrowth of epithelial structures) produced through the invasion process.
Results
The U-87 MG cell spheroids were harvested from the ultra-low attached U-shaped plate and placed on top of the hydrogel matrix (2D hydrogel coating invasion). Growth of the cell spheroids on both VitroGel® 3D and VitroGel® Hydrogel Matrix can be seen over a 7-day course (Figure 1). The cells on VitroGel 3D maintained the spheroid morphology with an expansion in size from day 1 to day 7 (Figure 1a; 1b).
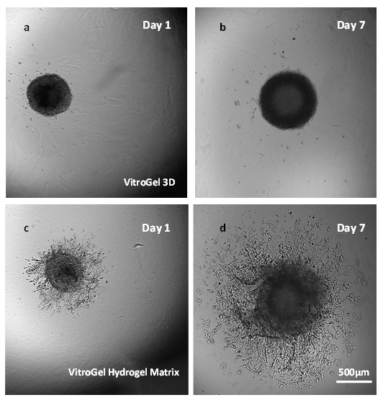
Figure 1. Growth of cell spheroids over time in the VitroGel 3D (a and b) and the VitroGel® Hydrogel Matrix (c and d).
However, the spheroid did not develop epithelial extensions characteristic of U-87 MG cells that would show the invasion of the cells through the hydrogel matrix. In contrast, the spheroids grown in VitroGel® Hydrogel Matrix produced not only significantly larger spheroids by day 7 but invading epithelial structures, demonstrating the clear cell penetration into the hydrogel matrix (Figure 1c; 1d). The live-dead assay of day-7 cells on VitroGel Hydrogel Matrix (Figure 2) revealed significant cell viability when this functional hydrogel was used for 3D spheroid invasion assay. Comparing the images of spheroids in VitroGel 3D and VitroGel Hydrogel matrix, it is important to consider the pathophysiology of cell-matrix interaction within a 3D microenvironment.
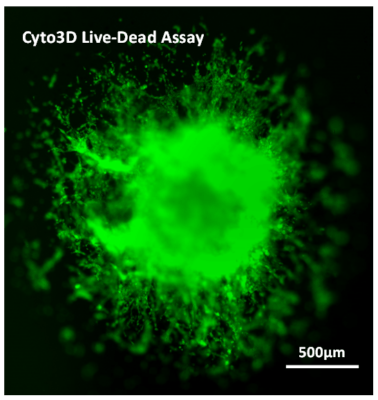
Figure 2. Live-dead assay of day-7 cells with VitroGel® Hydrogel Matrix
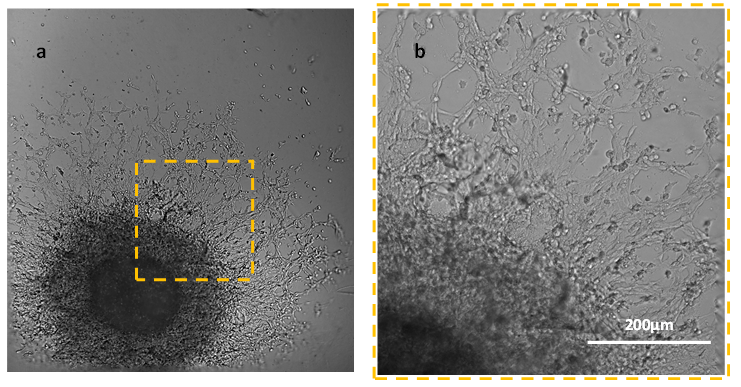
Figure 3. Day 7 of culture with VitroGel® Hydrogel Matrix. Panel b is the enlargement of the area in panel a.
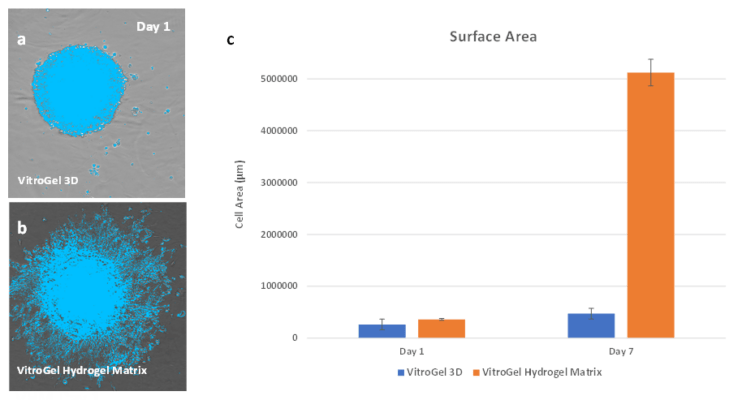
Figure 4. Surface area comparisons of culture growth in the two matrices.
Compared to the spheroid invasion by using the 2D hydrogel coating method, one could also directly add the hydrogel into the ultralow attached U-shape plate to encapsulate the U-87 MG cell spheroid in hydrogel matrix for 3D invasion. Similar to the results from the hydrogel coating method, the cell spheroids show great differences in the development of the invading structures depending on which hydrogel matrix was used (Figure 5). In VitroGel 3D, the cells maintain the spheroid structure without significant changing in the sizes or showing the cellular networking structure around the spheroid. In contrast, the spheroid grown in VitroGel Hydrogel Matrix paralleled the results from the hydrogel coating method. By day 1 the process of invasion in VitroGel Hydrogel Matrix had started, and by day 7 the malignant nature of the cells was visibly by the size and thickness of the spheroid, the surface area of the structure, and the development of cell-cell and cell-matrix interactions within the hydrogel.
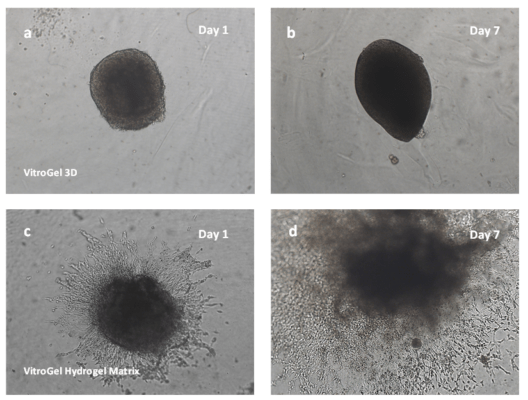
Figure 5. Images from direct attachment to U-shaped plate during the assay.
Discussion
Reference
- Hirschhaeuser, F., Menne, H., Dittfeld, C., West, J., Mueller-Klieser, W., & Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J Biotechnol, 2010. 148: p. 3–15.
- Pampaloni, F., Reynaud, E.G., & Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nature Rev Mol Cell Biol, 2007. 8(10): p. 839–845.Lim, G.J., Kang, S.-J., & Lee, J.Y. Novel invasion indices quantify the feed-forward facilitation of tumor invasion by macrophages. Sci Rep, 2010. 10:718.
- Blacher, S., Erpicum, C., Lenoir, B., Paupert, J., Moraes, G., Ormenese, S., … & Noel, A. Cell invasion in the spheroid sprouting assay: a spatial organisation analysis adaptable to cell behaviour. PloS One, 2014. 9(5): e97019.