Room temperature protocol. No ice bucket required. Room temperature stable protocol that can be completed in 30 minutes and can be adapted to laboratory automation.
VitroGel® High-Concentration Cell Invasion Assay Kits (Tunable)
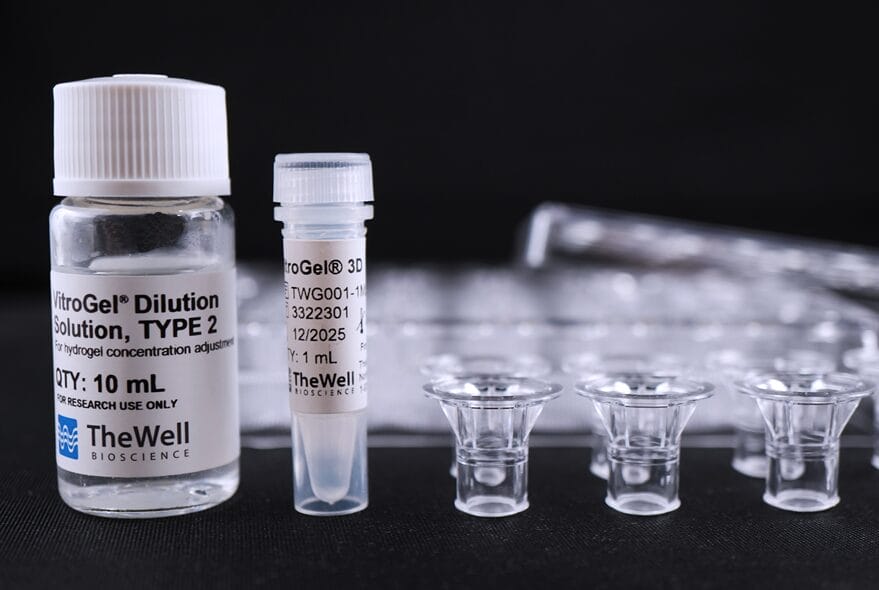
Xeno-free hydrogel system for consistent study of cell invasion. Kit includes choice of tunable functionalized hydrogel, 10mL VitroGel® Dilution Solution TYPE 2, and VitroPrime™ Cell Culture Inserts, 8 µm.
VitroGel® High-Concentration Cell Invasion Assay Kits (Tunable)
Your gateway for easy-to-use and consistent in-depth cell invasion studies.
Go beyond your traditional animal-based ECM for invasion studies with our xeno-free VitroGel® hydrogels and VitroPrime™ Cell Culture Inserts.

Full control of the ECM
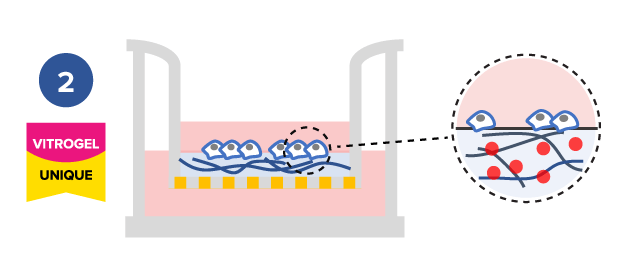
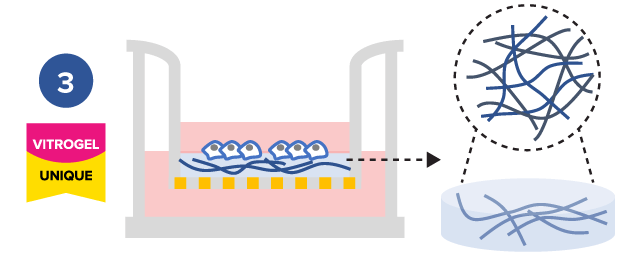
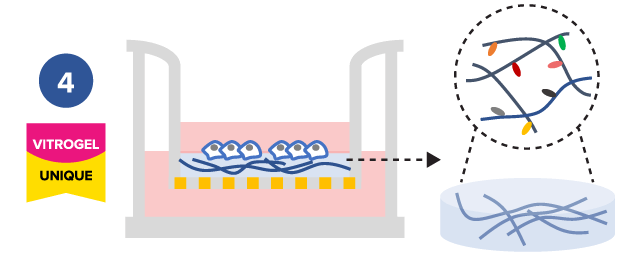
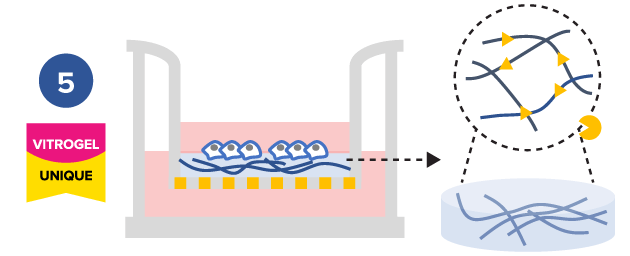
Tunable hydrogels allows adjustment of hydrogel strength, binding ligand, degradability, and supplement compositions to study the microenvironment’s influence on cell mobility.

Consistent results
No batch-to-batch variability. A defined system without unknown protein composition to ensure robust results.

Support barrier models
The system can be used to create in vitro complex microenvironment models.

Excellent image quality
The membrane does not stain when performing common staining for imaging (i.e., crystal violet).

Accurate evaluation of studies
Even pore sizes throughout the membrane provide accurate results when evaluating cell invasion.
Cell invasion, a vital process in various biological contexts, can be pivotal in embryonic development, immunosurveillance, and wound healing while also playing a concerning role in cancer metastasis. Traditional in vitro invasion assays have relied on animal-based extracellular matrices, which come with challenges like undefined components, batch-to-batch variability, and cumbersome temperature-sensitive protocols, thus leading to inconsistent and inaccurate studies.
The VitroGel® High-Concentration Cell Invasion Assay Kit (powered by the tunable VitroGel® High-concentration hydrogels) offers unmatched flexibility, allowing researchers to fully customize the hydrogel matrix—adjusting mechanical strength, binding ligands, and ECM components. The kit includes our high-quality VitroPrime™ Cell Culture Inserts, allowing more accurate and consistent invasion and migration studies than animal-based ECM. No other product enables such a deep exploration of cell mobility and invasion studies, providing researchers with the precision and control needed to push the boundaries of their experiments like never before.
If you just need a simple synthetic hydrogel system to replace animal-based ECM for most traditional cell mobility studies, our ready-to-use VitroGel® Cell Invasion Assay Kit is an optimal choice! This easy-to-use hydrogel offers a direct substitute for animal-based ECM while also allowing researchers to fine-tune their experiments by adjusting supplements and key factors within the hydrogel matrix. Learn more about our ready-to-use VitroGel® Cell Invasion Assay Kit >
Specifications
| Use | Cell Invasion, cell migration, co-culture, barrier model |
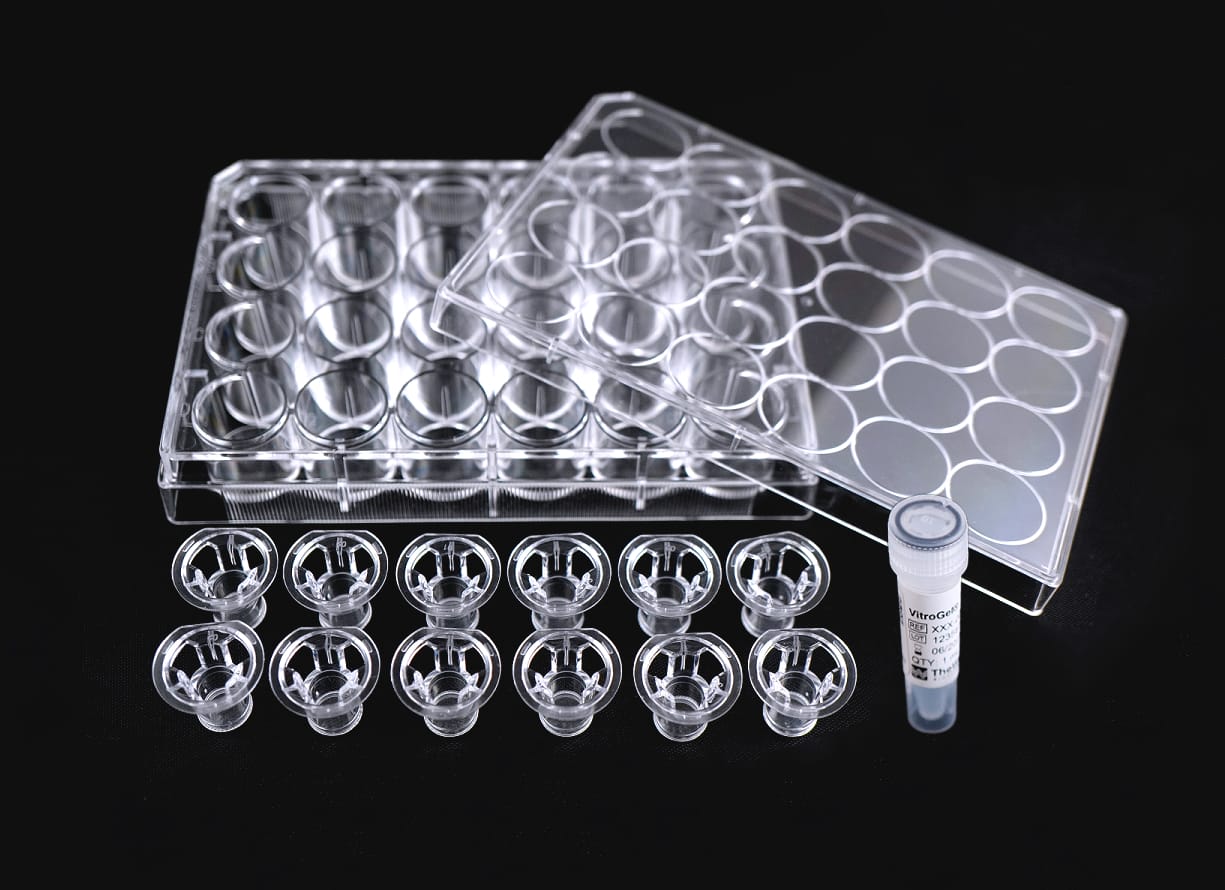
| Inserts | VitroPrime™ Cell Culture Inserts 8 µm, PET, Transparent, Sterile. |
| Kit Contents | VitroGel® High Concentration Hydrogels VitroGel® Dilution Solution, TYPE 2 VitroPrime™ Cell Culture Inserts 8 µm, PET, Transparent, Sterile. |
| Formulation | Xeno-free, functional hydrogel |
| pH | Neutral |
| Storage | Store hydrogel at 2-8°C. Ships at ambient temperature |
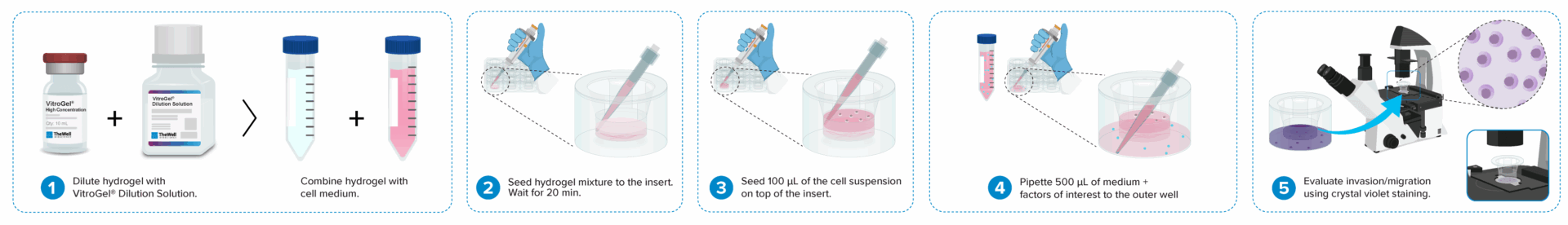
Cell invasion culture process in 30 minutes.
Work confidently at room temperature. No ice bucket required. VitroGel® High-Concentration Cell Invasion Assay Kits are ready to use. There is no cross-linking agent required.Learn how gelation works >
Type of studies capable with VitroGel® High-Concentration Cell Invasion Assay Kits (Tunable)
Traditional invasion assay with chemoattraction from outer well
Study effect of cytokine/supplement of hydrogel matrix on cell mobility
Study the effect of different hydrogel mechanical strengths on cell mobility
Study the effect of hydrogel functional ligands on cell mobility
Study the effect of hydrogel degradability on cell mobility
Study the effect of cytokine/ supplement of hydrogel matrix on cell mobility
Comparison of VitroGel® versus traditional animal-based ECM for cell invasion studies
VitroGel® High-Concentration Cell Invasion Assay Kit | Traditional assay with animal-based ECM |
|
Operation temperature | Room temperature | 2-8 °C |
Set up time | 30 mins | 2 hours + |
Control compounds of outer well | ✔ | ✔ |
Consistent results | ✔ | ― |
Control key compounds in hydrogel | ✔ | ― |
Control mechanical strength of hydrogel | ✔ | ― |
Study functional ligands of hydrogel | ✔ | ― |
Control hydrogel degradation | ✔ | ― |
High-throughput / Lab automation | ✔ | ― |
Protocols and Resources
![]() VitroGel® 3D Cell Invasion Assay Kit
VitroGel® 3D Cell Invasion Assay Kit
![]() VitroGel® RGD Cell Invasion Assay Kit
VitroGel® RGD Cell Invasion Assay Kit
![]() VitroGel® IKVAV Cell Invasion Assay Kit
VitroGel® IKVAV Cell Invasion Assay Kit
![]() VitroGel® YIGSR Cell Invasion Assay Kit
VitroGel® YIGSR Cell Invasion Assay Kit
![]() VitroGel® COL Cell Invasion Assay Kit
VitroGel® COL Cell Invasion Assay Kit
![]() VitroGel® MMP Cell Invasion Assay Kit
VitroGel® MMP Cell Invasion Assay Kit
Video Protocols | Webinars | Application Notes
Research Highlights
Data and References

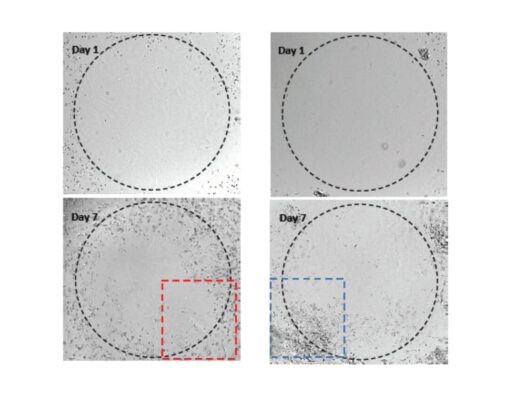
Study the invasion of U87-MG Glioblastoma cells on hydrogel with different mechanical strengths
- Tunable Cell Invasion Assay Kit: VitroGel® RGD Cell Invasion Assay (Cat # IA-HC003-4P)
- Insert: VitroGel® RGD hydrogel at 1:1, 1:3 and 1:5 dilution ratios
- Outer well: No serum v.s. 20% FBS
- Cell Incubation time: 24 hrs
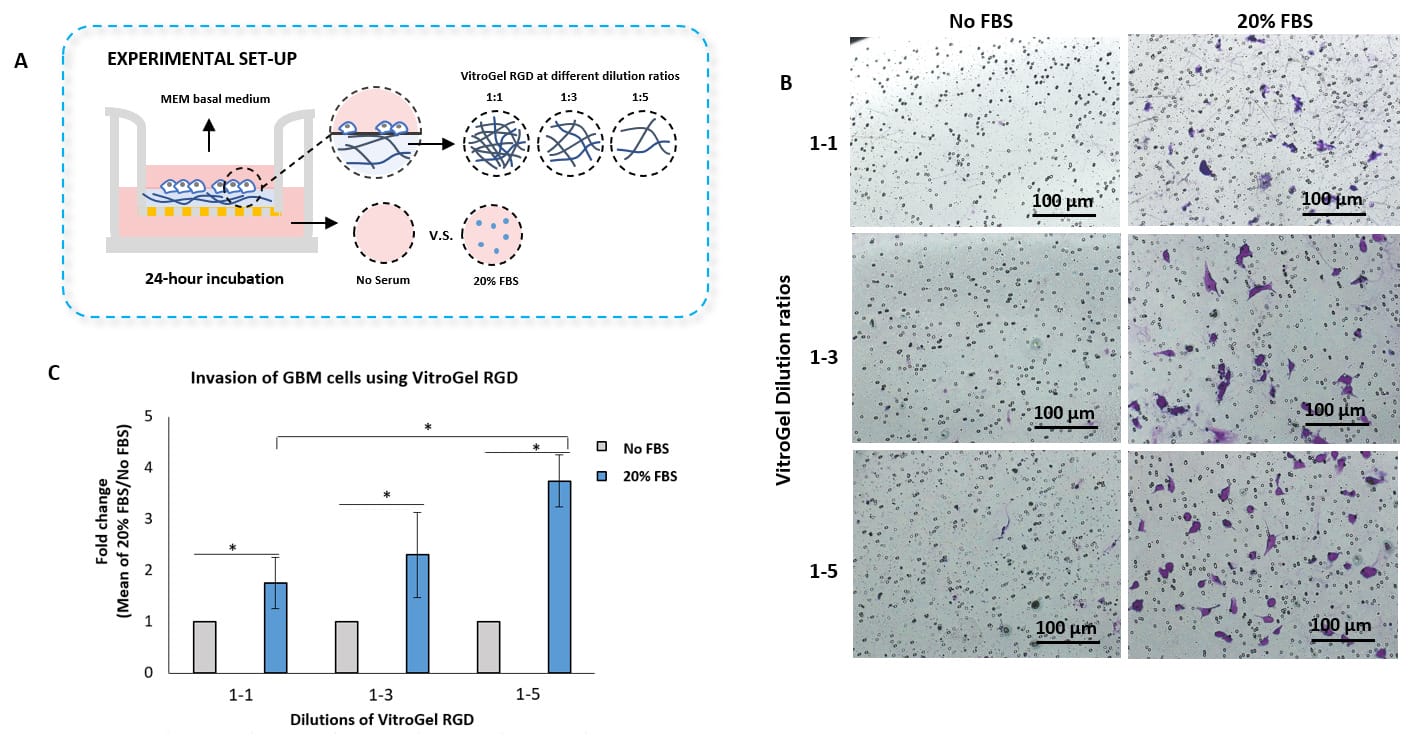
Invasion of U87-MG glioblastoma (GBM) cells using VitroGel® RGD high-concentration hydrogel.
VitroGel® RGD was diluted with VitroGel® Dilution Solution at the following ratios: 1:1, 1:3, and 1:5. The hydrogel mixture was further diluted with MEM 1X at a 4:1 ratio. The hydrogel mixture was added to the inserts and allowed to solidify for 20 minutes at RT. U87-MG glioblastoma cells (3.8 x 104 cells/insert) were resuspended in a 100 µl of serum-free medium and added to the inserts. 500 µl of either serum-free medium or MEM 1X supplemented with 20% FBS were added to the outer wells. The cells were incubated for 24 hours at 37°C. Then, cell invasion was visualized by performing crystal violet staining and imaging. Images were obtained with the Zeiss microscope at a 10X magnification. *p<0.05 (Please check the Product page of VitroGel® RGD for mechanism strengths at different dilutions.
![]()
Effect of hydrogel functional ligands and degradability on cell mobility
The tunable VitroGel® high-concentration hydrogels were designed based on different hydrogel properties, e.g. the modification of functional ligands and degradability of hydrogel matrix. The tunable VitroGel® system provides a novel tool to study the effects of hydrogel biochemical properties on cell mobility.

Study the effect of functional ligands and degradability of hydrogel matrix on cell mobility
- Tunable Cell Invasion Assay Kits:
VitroGel® 3D Cell Invasion Assay Kit (Cat# IA-HC001-4P)
VitroGel® MMP Cell Invasion Assay Kit (Cat# IA-HC010-4P)
VitroGel® RGD Cell Invasion Assay Kit (Cat# IA-HC003-4P) - Insert: VitroGel® 3D, MMP, or RGD hydrogel at 1:3 dilution ratio
- Outer well: No serum v.s. 20% FBS
- Cell Incubation time: 48 hrs
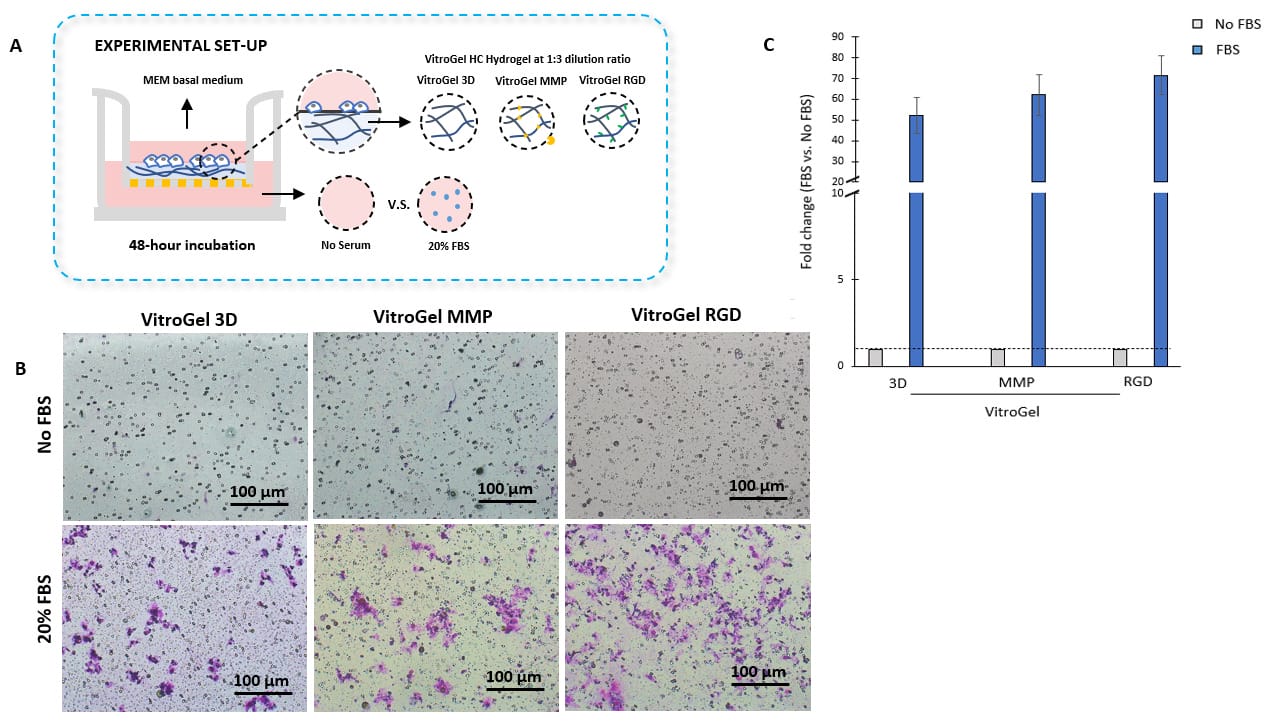
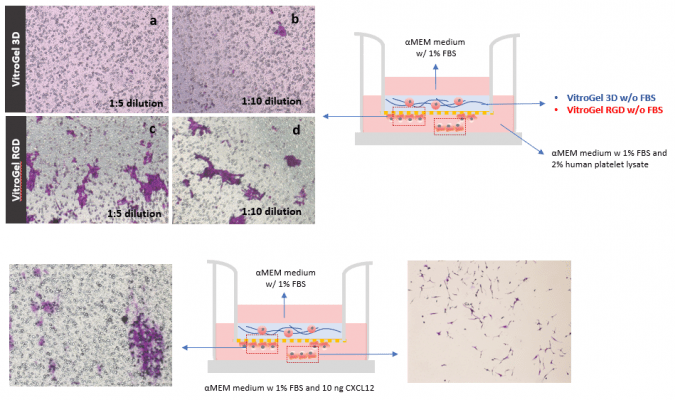
Bio-functional ligands inside hydrogel influence U87-MG glioblastoma cell invasion.
A. Schematic representation of experimental setup. Hydrogels were diluted with VitroGel®dilution solution in a 1:3 ratio, placed in the insert, and allowed to solidify for 20 mins. B. Light microscopy images showing U87-MG glioblastoma cell invasion through VitroGel® high-concentration hydrogels with different bio-functional ligands. Images were obtained with a Zeiss microscope at a 10X magnification. C. Fold change of cell invasion in the FBS group relative to the No FBS group for each hydrogel.
![]()
Effect of both cytokine and functional ligands in hydrogel matrix on cell mobility
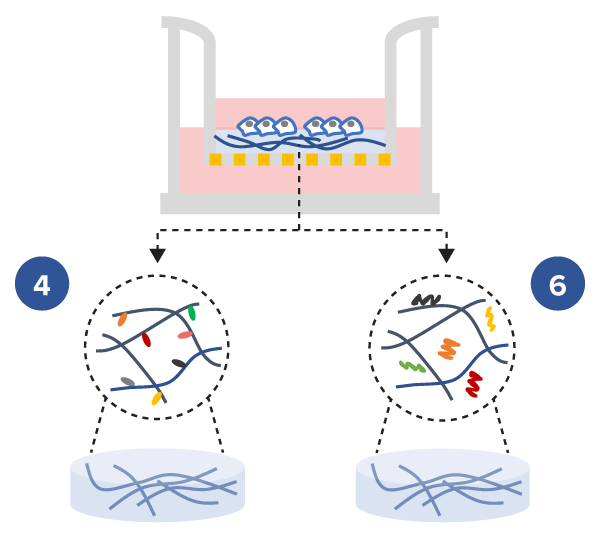
The defined composition and the multiple functional ligand modifications of the tunable VitroGel® high-concentration hydrogels allow researchers to create a complex microenvironment with full control of the hydrogel supplement content e.g. chemokines, cytokines, growth factors, etc., to study cell mobility.

Study the effect of both cytokine and functional ligands of hydrogel matrix on cell mobility
- Cell Invasion Assay Kits:
VitroGel® 3D Invasion Assay Kit (Cat# IA-HC001-4P)
VitroGel® RGD Cell Invasion Assay (Cat# IA-HC003-1P) - Insert: VitroGel® 3D and VitroGel® RGD hydrogels at 1:3 dilution ratio with TGF-β1 v.s. without TGF-β1
- Outer well: No serum v.s. 20% FBS
- Cell Incubation time: 48 hrs
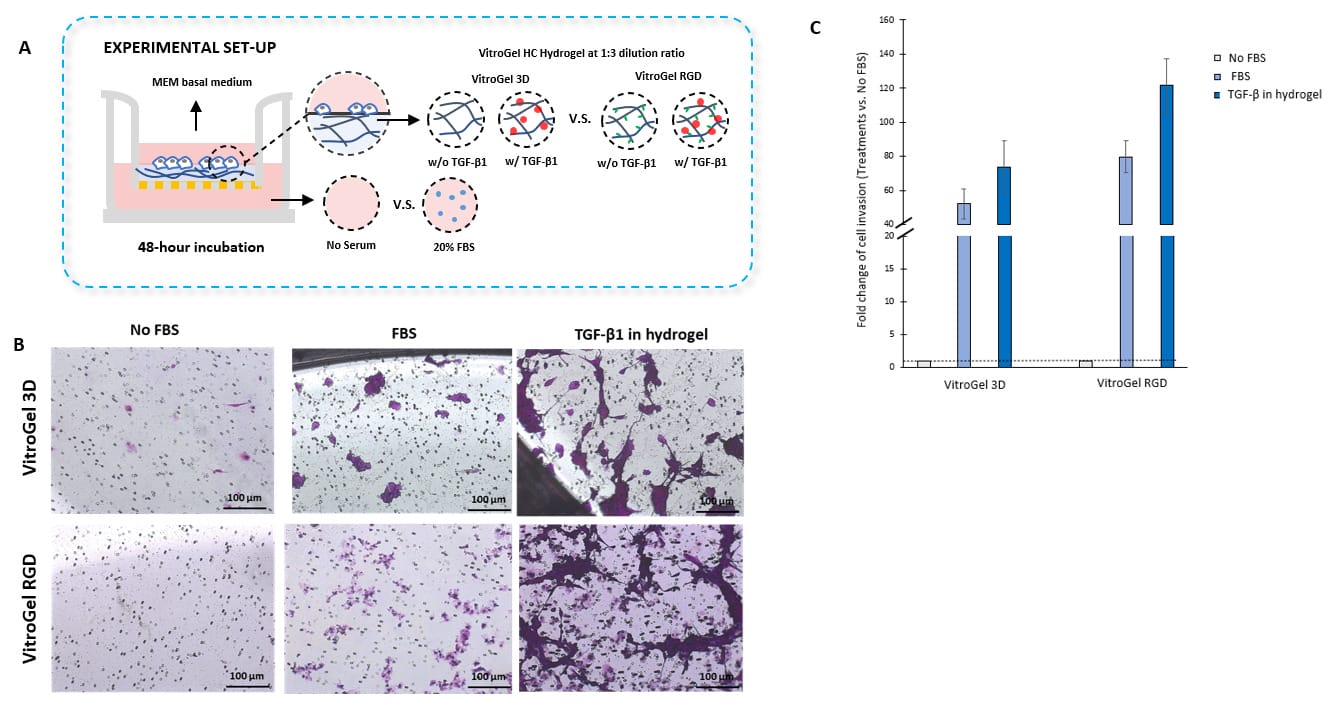
TGF-β1 inside VitroGel® 3D and VitroGel® RGD facilitates U87-MG glioblastoma cell invasion.
A. Visual representation of experimental setup. Cultures were incubated for 48 hours B. Microscopy images demonstrating U87-MG glioblastoma cell invasion through VitroGel® 3D and RGD. Each hydrogel was diluted with VitroGel® Dilution solution in a 1:3 ratio and then combined with MEM 1X or MEM 1X with TGF-β1 (30 ng/mL) in a 4:1 ratio. Images were obtained with a Zeiss microscope at a 10X magnification. C. Fold change of cell invasion in the TGF-β1 in hydrogel and FBS groups relative to the No FBS group for each hydrogel. The No FBS group was normalized to 1.
References/Publications
- Yang, L., Shi, J., Zhong, M., Sun, P., Zhang, X., Lian, Z., Yin, H., Xu, L., He, G., Xu, H., Wu, H., Wang, Z., Miao, K., & Huang, J. (2024). NXPH4 mediated by m5C contributes to the malignant characteristics of colorectal cancer via inhibiting HIF1A degradation. Cellular & Molecular Biology Letters, 29(1). https://doi.org/10.1186/s11658-024-00630-5
- Daulagala, A. C., Cetin, M., Nair-Menon, J., Jimenez, D. W., Mary Catherine Bridges, Bradshaw, A. D., Sahin, O., & Antonis Kourtidis. (2024). The epithelial adherens junction component PLEKHA7 regulates ECM remodeling and cell behavior through miRNA-mediated regulation of MMP1 and LOX. BioRxiv (Cold Spring Harbor Laboratory). https://doi.org/10.1101/2024.05.28.596237
- Xu, L., Yang, L., Zhang, D., Wu, Y., Shan, J., Zhu, H., Lian, Z., He, G., Wang, C., & Wang, Q. (2024). Multi-omics analysis reveals the unique landscape of DLD in the breast cancer tumor microenvironment and its implications for immune-related prognosis. Computational and Structural Biotechnology Journal. https://doi.org/10.1016/j.csbj.2024.02.016
- Liu, M., Yu, B., Tian, Y., & Fan, L. (2024). Regulatory function and mechanism research for m6A modification WTAP via SUCLG2-AS1- miR-17-5p-JAK1 axis in AML. BMC Cancer, 111938–111938. https://doi.org/10.1016/j.jbiomech.2024.111938
- Naik, N., Bharadwaja Vadloori, Suresh Poosala, Srivastava, P., Coecke, S., Smith, A. J., Akhtar, A., Roper, C., Radhakrishnan, S., Balaji Bhyravbhatla, Damle, M., Venkat Koushik Pulla, Johannes Hackethal, Reyk Horland, Li, A. P., Pati, F., Manu Smriti Singh, Occhetta, P., Rohit Bisht, & Dandekar, P. (2023). Advances in Animal Models and Cutting-Edge Research in Alternatives: Proceedings of the Third International Conference on 3Rs Research and Progress, Vishakhapatnam, 2022. Atla-Alternatives to Laboratory Animals, 51(4), 263–288. https://doi.org/10.1177/02611929231180428
- Shamloo, B., Kumar, N., Owen, R. H., Reemmer, J., Ost, J., Perkins, R. S., & Shen, H.-Y. (2019). Dysregulation of adenosine kinase isoforms in breast cancer. Oncotarget, 10(68). https://doi.org/10.18632/oncotarget.27364
| Assay Kit | VitroGel Cell Invasion Assay Kit, VitroGel 3D Cell Invasion Assay Kit, VitroGel RGD Cell Invasion Assay Kit, VitroGel IKVAV Cell Invasion Assay Kit, VitroGel YIGSR Cell Invasion Assay Kit, VitroGel COL Cell Invasion Assay Kit, VitroGel MMP Cell Invasion Assay Kit |
|---|---|
| Assay Kit Configuration | 1 mL + (12) VitroPrime™ Cell Culture Inserts, 4 mL + (48) VitroPrime™ Cell Culture Inserts |