Application Notes
Characterizing the Biophysical Properties of a Tunable Hydrogel System for 3D Cell Culture
Poster Presentation
TheWell Bioscience Inc.
Nana Fatima Haruna, Ziwen Vivian Wang, John Huang
TheWell Bioscience, Inc., North Brunswick NJ 08902
Abstract
The hydrogel-based 3D cell culture model more accurately depicts the microenvironment of cells in vivo than the traditional 2D cell culture system, which enables cells to grow, develop, operate, and produce feedback as if they were in a living organism. As we continue to learn more about cell growth in a 3D microenvironment, we come to understand that, like the 2D cell culture system, the cell-cell communication, and cell-matrix interactions rely on many variables of the hydrogel biophysical properties including stiffness, density, porosity, binding ligands, and composition. However, because the current animal-derived hydrogel systems struggle with the batch to batch consistency, while the final properties of most hydrogel systems are depended on several key factors such as culture medium, cell density, and supplement compounds there is a lack of the thorough study on the biophysical properties of current hydrogel-based 3D cell culture products. Here, we used a xeno-free, tunable hydrogel system, VitroGel®, to characterize the hydrogel rheological properties in response to ionic molecules, compositions of cell culture mediums, and supplements such as glucose and serum. The tests covered different stages of the hydrogel (stable, and injection) at various dilution and mixing ratios. The molecular diffusion properties of hydrogels at different concentrations and formation states were also characterized with phenol red, trypan blue, BSA and IgG. Glioblastoma cancer cells (U87MG) and bone marrow stromal cell (OP9) were cultured in the 3D hydrogel to evaluate the intercellular characters in response to the different hydrogel conditions. The data indicated that the VitroGel system can be a platform with defined and adjustable biophysical properties to support drug screening and toxicology studies, tissue engineering, regenerative medicine, and other basic cell biological studies.
Materials and Methods
Rheology: Single frequency oscillation time sweeps were performed using a Malvern Kinexus Pro+ dynamic rheometer with a 20 mm parallel plate rough surface geometry. For the gel formation tests, 190 ul of the sample mixture was loaded on a 30 mm lower geometry plate with 0.5 mm gap size. The rheometer was set to perform a 1 Hz time sweeps for 30 mins. Repeated amplitude sweep (shear thinning and recovery) tests were also performed on the samples.
Cell Culture: U-87 MG cells and OP9 GFP cells were cultured in Alpha MEM α MEM) supplemented with 10 fetal bovine serum, 1 X Pen Strep and 1 X Amphotericin B The cells were prepared in 3-D cell culture using VitroGel (User Handbook Available Click Here).
How does VitroGel work?
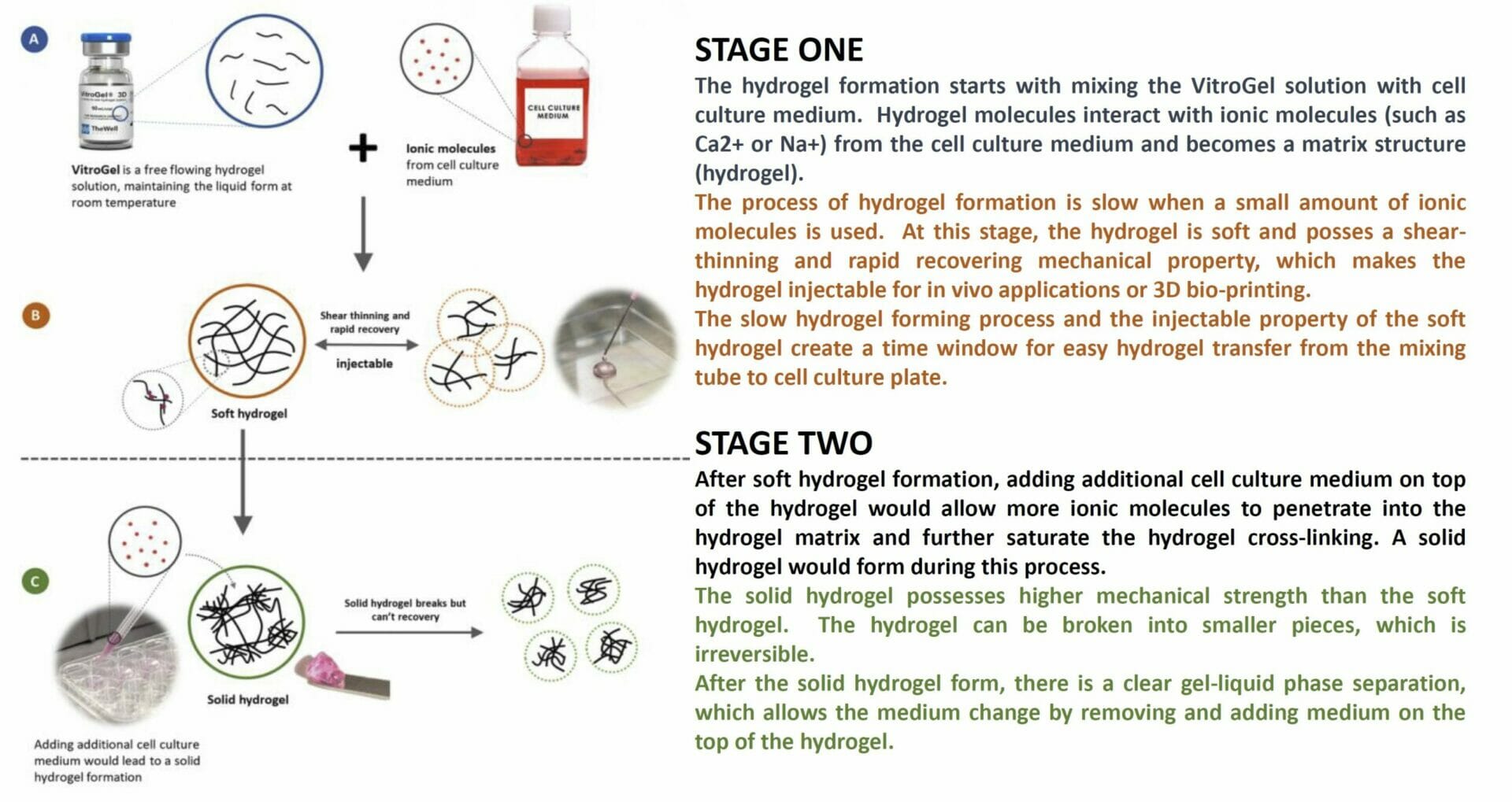
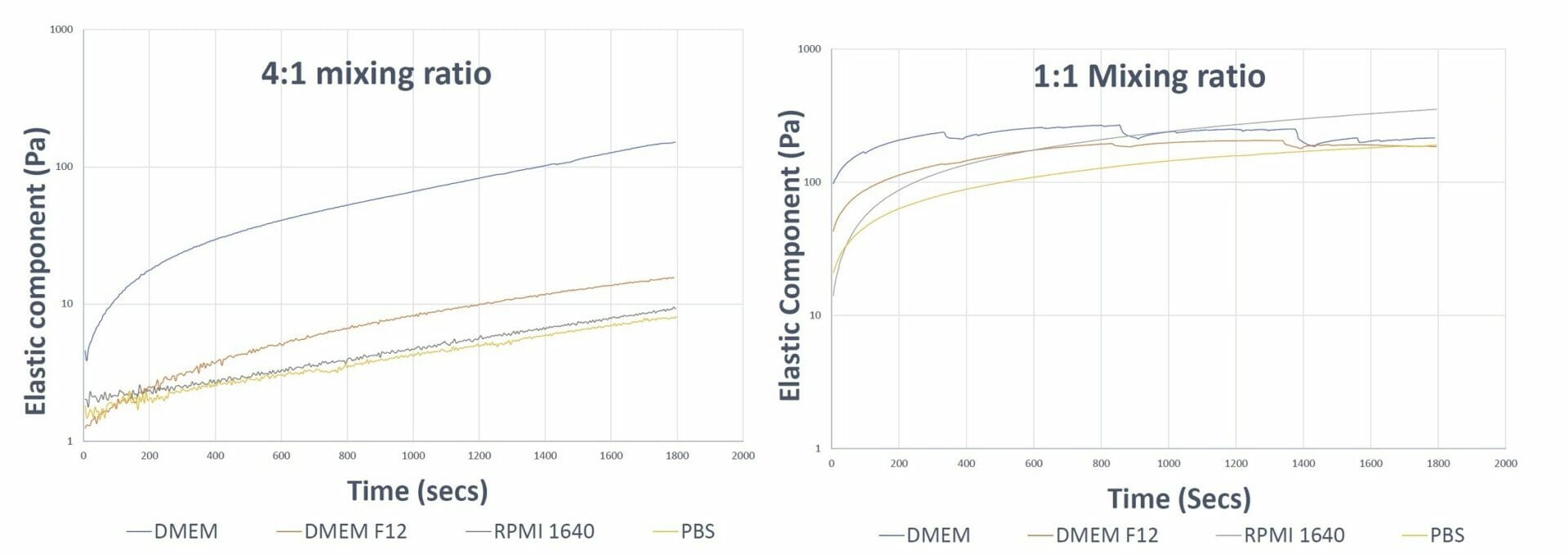
Figure 1. Gel formation curves of VitroGel 3D hydrogel at different mixing ratios The 4:1 mixing ratio graph shows smooth formation in all the samples and faster hydrogel formation in the sample prepared with DMEM The 1:1 mixing ratio curve shows a dip in the DMEM and DMEM F12 curve, indicating fast aggregation of the hydrogel which disturbs the integral matrix formation The 1:1 mixing ratio graph shows smooth curves for the RPMI and PBS samples as a result of their lower ionic concentrations.
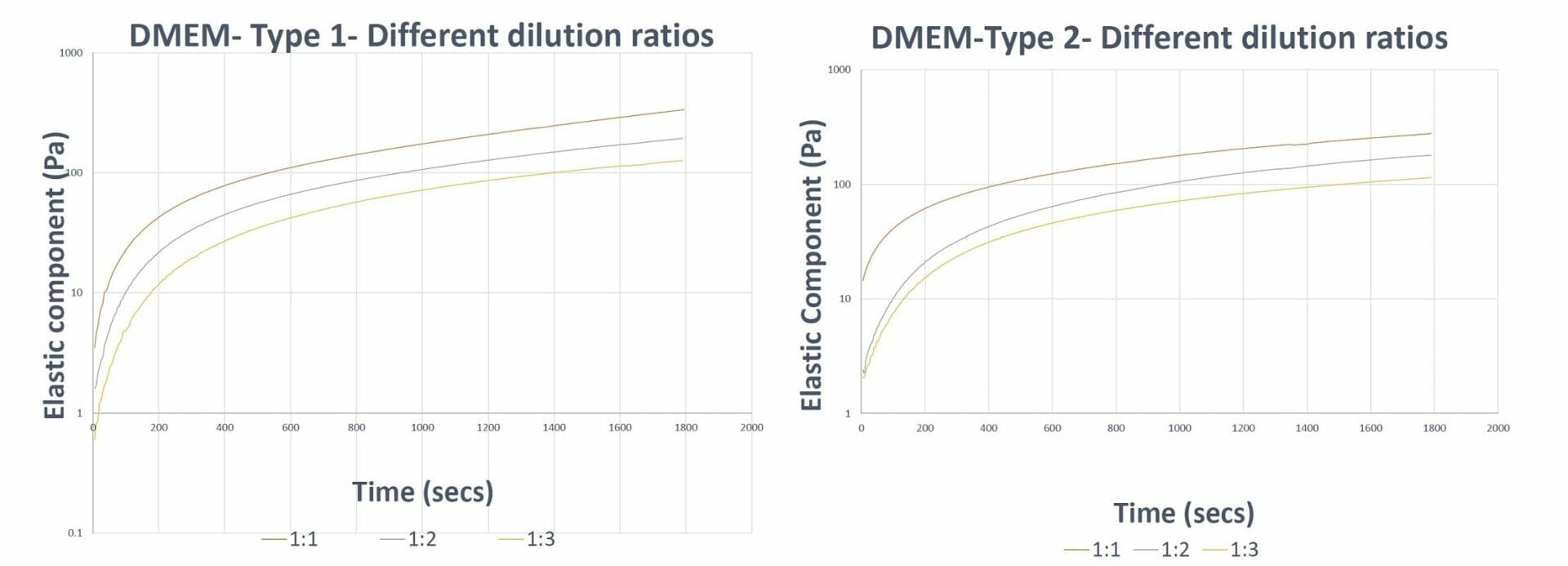
Figure 2. Gel formation curves of VitroGel 3D hydrogel prepared with VitroGel Dilution Solutions The results show the capability of VitroGel Dilution Solutions to aid in the balancing of hydrogel formation (keep the trend/slope of curves similar) at different dilution levels.
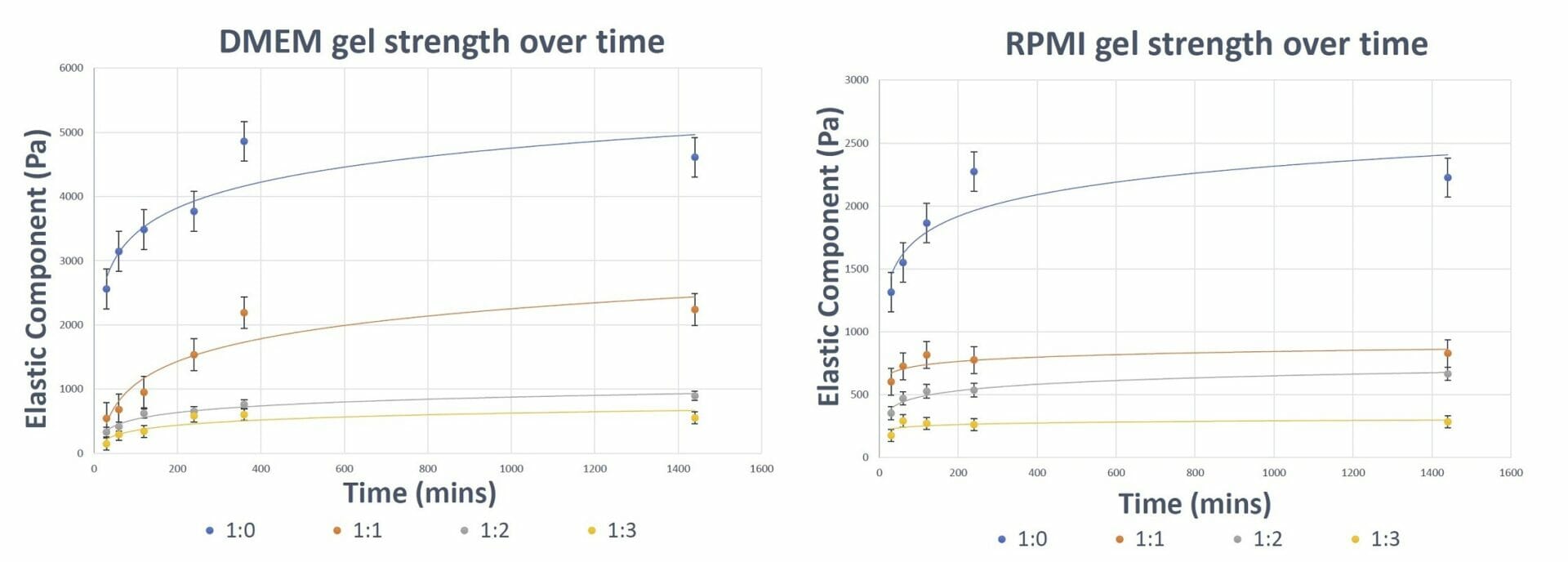
Figure 3. Gel Strength of VitroGel Hydrogel over time The results show the ability of VitroGel hydrogel to form and attain its final gel strength within 4-6 hours (depending on the type of medium used).
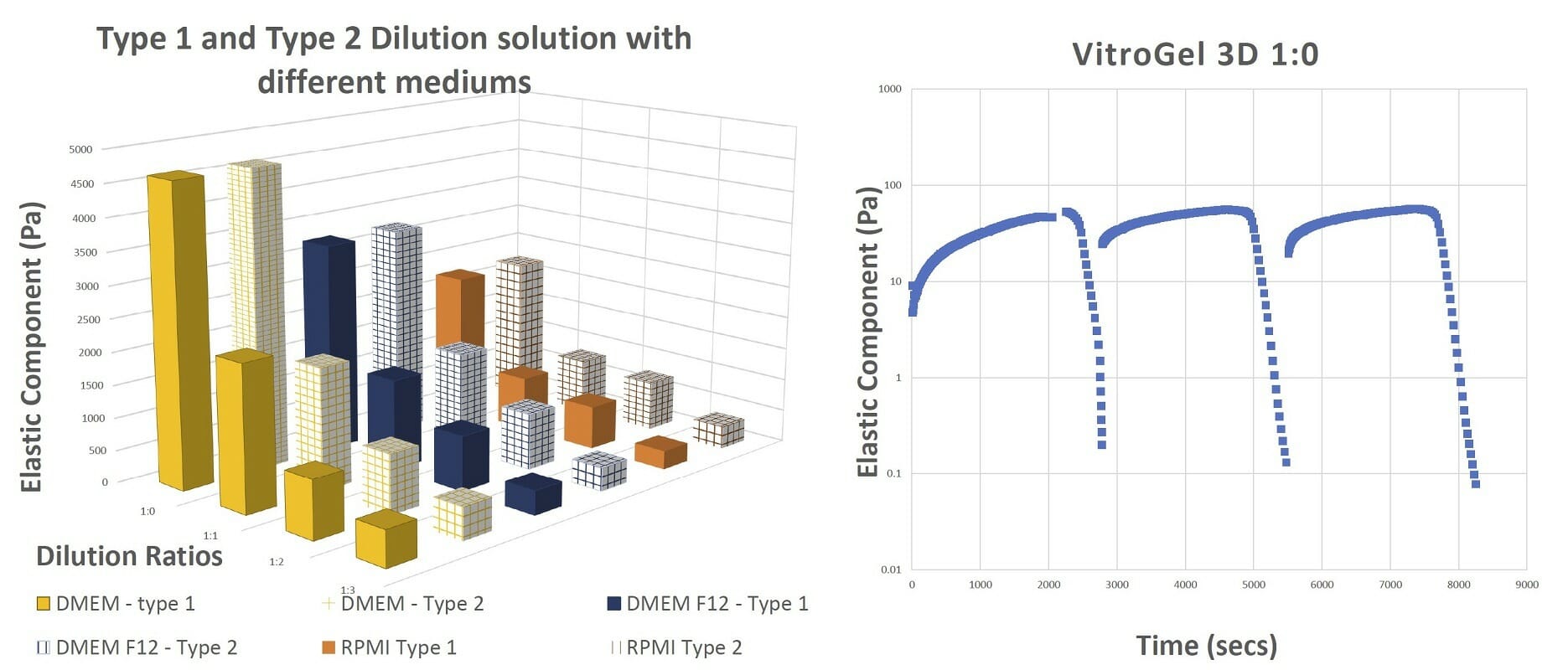
Figure 4. The final gel strengths, shear thinning and rapid recovery of VitroGel hydrogel The results of the shearing table test from 0.1% to 100% shear strain show that the hydrogel recovers after every cycle of shear strain.
3D Cell Culture on VitroGel System
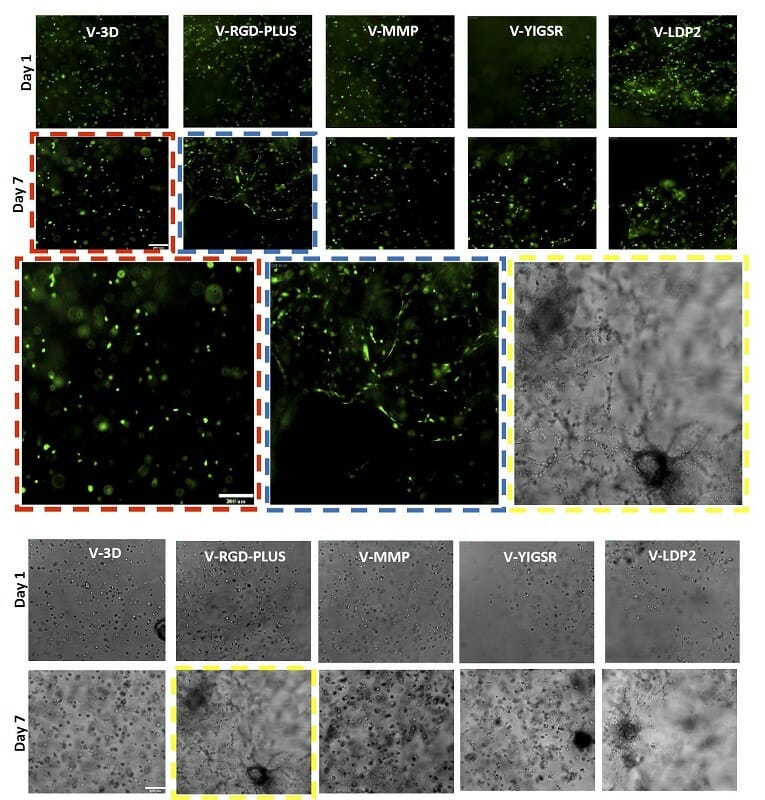
Figure 5. OP9 GFP (top) and U87MG (bottom) cells in different VitroGel Hydrogels. The results show V-RGD-PLUS and V-LDP2 support OP9 fibroblast morphology and U87 epithelial morphology, however, V-3D supports cell growth but not the characteristic morphology.
Molecular diffusion Properties of VitroGel
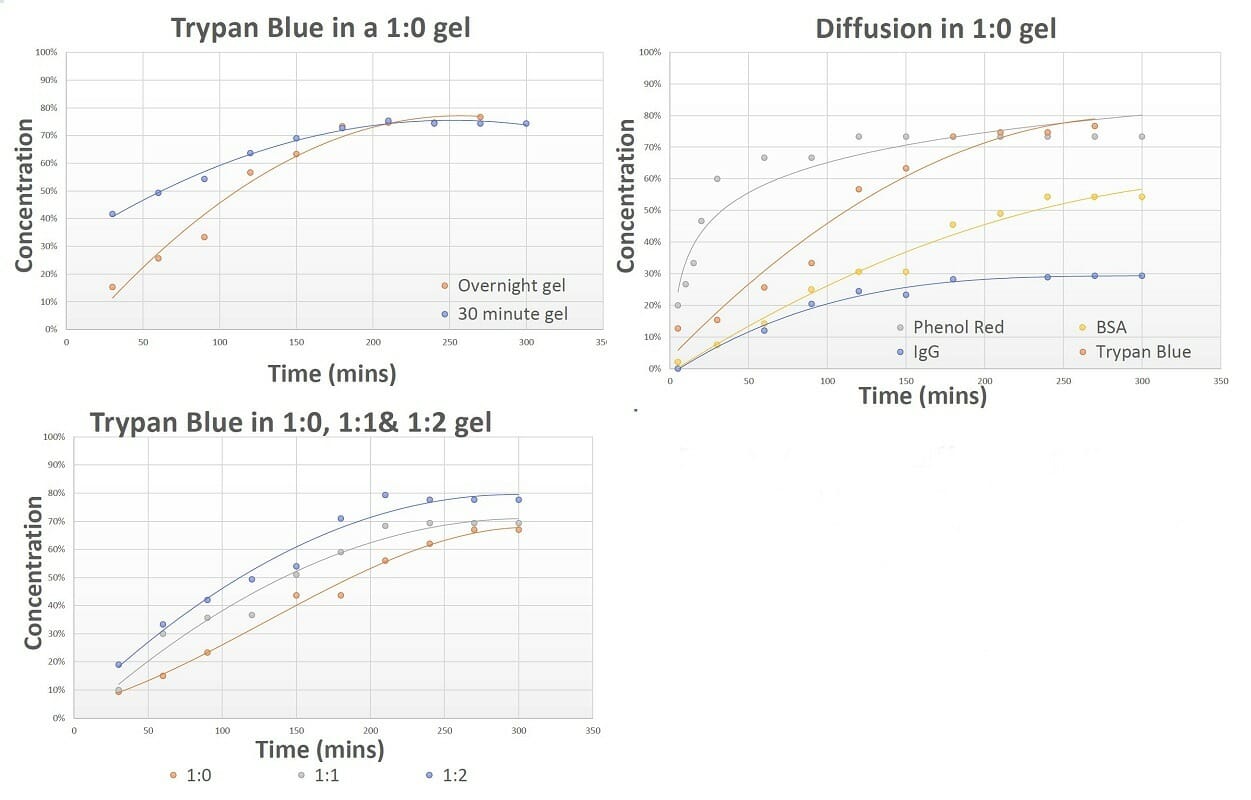
Figure 6. The diffusion patterns of different molecules through VitroGel hydrogel. The results show VitroGel hydrogel is suitable for drug screening and cell therapy applications as it allows for free movement of molecules in and out of the gel
- VitroGel® is a ready-to-use, xeno-free system that transforms into a tunable hydrogel matrix by simply mixing with cell culture medium.
- By adjusting the biophysical properties and modifying with functional ligands, VitroGel can closely mimic the natural extracellular matrix (ECM) environment to promote cell-cell and cell-matrix interactions.


