Application Notes
Cell Line-Derived Xenograft Using Xeno-Free VitroGel System in Comparison to Animal-based Extracellular Matrix
Application Note
Shreya Krishnaswamy1, Minqi Huang2, Luke Tsai2, John Huang1
1 TheWell Bioscience Inc., North Brunswick, NJ
2 In Vivo Pharmacology, HD Biosciences, a Wuxi AppTec company, San Diego, CA

Introduction
Cancer progression is highly individualized. The mechanism is specific to each animal species, making oncology research one of the most challenging areas in biosciences. Recent advances that focus on the individualized nature of disease progression have been very promising; for example, Patient-Derived Xenografts (PDX) models, in which immunodeficient animals have human-derived tumor tissues embedded in them, are revealing some key diagnostic and therapeutic information. Similarly, Cell Line-Derived Xenograft (CDX) models, in which specific cell lines are used for the engrafting, have proven themselves to be significant tools by providing a powerful platform to study tumor growth and progression, thereby advancing cancer therapies 1,2. CDX models also serve as an important intermediate in the drug discovery process. Hydrogel systems such as from a widely known animal-based extracellular matrix (AB-ECM) have been used for decades, acting as a delivery system for in vivo applications. However, the presence of innumerable undefined compounds leads to inconsistency between the AB-ECM batches. Some practical challenges, such as labile cell preparation, graft rejection, fear of clumping, or other viscosity issues, remain in the use of current animal-derived matrices. AB-ECM’s temperature-sensitive attribute can also lead to hindrances during product usage. Moreover, the manufacturing cycle of AB-ECM is very slow (4 months on average), which causes the capability issue to support the increasing demands of xenograft studies. To help researchers advance their preclinical studies in a more timely and efficient manner, this application note discusses the exploration of TheWell Bioscience’s xeno-free, synthetic, and room temperature stable VitroGel® system in a CDX model 3. This application note specifically details the comparison between using AB-ECM versus VitroGel in CDX mouse models to study tumor characteristics. Some of the most widely used cell types in CDX models were chosen for this study. These spanned over several human tissue sources, including the prostate (PC3), cervical (HeLa), breast (HCC1806), colon (HCT116), lung (Calu-6), and pancreas (PANC1). Each line was inoculated into the mice after mixing with AB-ECM or VitroGel to obtain a comparative study of the progression of the tumor size expressed in volume.
About TheWell Bioscience’s VitroGel system for Xenograft
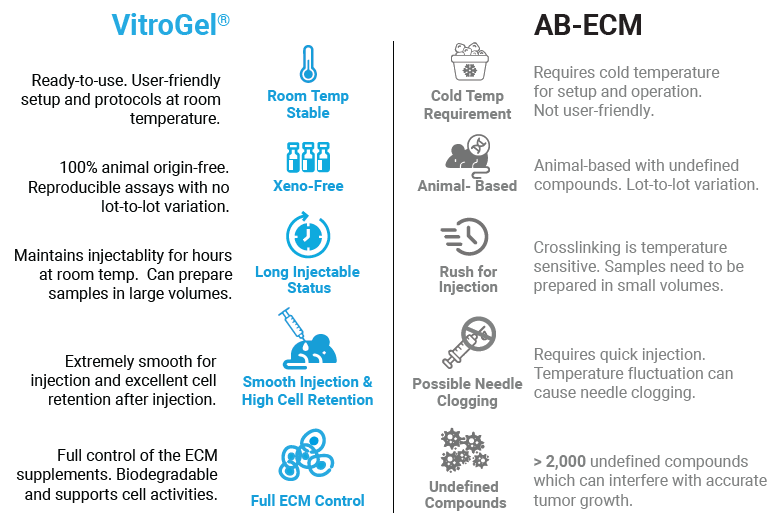
TheWell Bioscience’s VitroGel system acts as an excellent injectable delivery system that behaves as an efficient carrier of the diverse range of cell types and tissues for xenograft. Apart from being animal-free and completely synthetic, its strong bio-functional properties mimic the tissue microenvironment to support rapid cell growth. The product tags along certain key attributes and benefits that give it an edge over AB-ECM and other matrices, securing it as a superior alternative (Figure 1). Below are a few of the key benefits:
- Support over 150 cell types for both in vitro and in vivo applications
- Suitable for CDX model and PDX model with different host types (NSG mouse, nude mouse, SRG rate, and more) and multiple injection sites (subcutaneous, orthotopic, and more)
- Reproducible Assays – defined synthetic hydrogel composition without unknown proteins with no lot-to-lot variation
- Room temperature stable – easy protocol steps at room temperature
- Long-term injectable status – maintains injectability for hours at room temperature (can prepare the sample in bulk)
- Smooth injection without the issue of needle clogging
- Excellent cell retention after injection
- Always in stock, no supply chain issues
About Wuxi AppTec’s Animal Study Services
HD Biosciences, a WuXi AppTec company, offers a full spectrum of high-quality, timely, and cost-effective disease drug discovery, predictive biomarkers, companion diagnostics discovery, and In Vivo pharmacologic services for cancer biology needs. Leveraging the company’s cutting-edge technology, HD Biosciences has built a comprehensive platform for Immuno-Oncology, Antibody-drug conjugates, mRNA-LNP, and small molecules drug discovery to accelerate each client to grow their Oncology portfolio. The In Vivo pharmacology team provides all cancer model developments, non-GLP Pharmacokinetic / Pharmacodynamic, toxicology, and efficacy studies. The Ex Vivo team also offers an in-depth mechanism of action studies and supports translational predictive biomarker development.
HD Bioscience utilizes cell line-derived Xenograft (CDX) and syngeneic models to support target validation, translational medicine, and integrated drug discovery effort for efficacy and safety evaluation.
- CDX Model: Over 130 models, isogenic inducible, more under development
- Syngeneic Model: Over 50 validated models, more under development
- Orthotopic/Metastatic Model: Bladder, colon, liver, breast, kidney, lung, leukemia, pancreas, and skin
- Chemical-induced Model: nSTZ and high-fat diet-induced HCC model
- Humanized Model: NOD-SCID NSG/NCG mice with human PBMC engrafted
Materials and Methods
Overview of cell lines and hydrogel systems
The tumor development study was conducted in 60 athymic nude CDX mice models. The age of the mice chosen ranged from 6–8 weeks at the time of inoculation. All mice for this experiment were supplied by Charles River Laboratories. The VitroGel Hydrogel Matrix (TheWell Biosciences; catalog number: VHM01) and AB-ECM acted as the delivery system for the injection of specific tumor cell lines. Six cell lines, PC3, HCT-116, Calu-6, HCC1806, HeLa, and PANC1, were inoculated into the mice after mixing with either VitroGel or AB-ECM in a paired fashion, with five mice per treatment being studied (Table 1). These cell lines spanned several human tissue sources, including prostate, colorectal, lung, ovary, pancreas, and breast (Table 1). Mice and their tumors were observed over a period expressed in days for the treatment group with Hydrogel and AB-ECM to compare the time needed for each to reach the predetermined tumor size within a time frame of 2–10 weeks.
Cell culture media and handling of VitroGel versus AB-ECM
Cells were sub-cultured in specific media (Table 1), and those growing in the exponential phase were harvested. Cells were then resuspended in PBS without the addition of more supplements. After one round of filtration and cell counting, the cell suspension was followed by another round of centrifugation at 335 x g RCF, after which PBS was used to resuspend the cells to get 2X of desired cell concentrations.
Following this step, the protocols used for VitroGel versus AB-ECM were initiated with slight variations. For the methodology using VitroGel, the product was first brought to room temperature. The cell suspension (2X) was mixed with VitroGel at a ratio of 1:1 (v/v). After being transferred to the syringe, this mixture was left at room temperature for 10–15 minutes, during which time crosslinking starts to stabilize before injection. The injection volume was either 100 μL or 200 μL, depending on the cell type, to obtain the required cell density (1 x 106, 2 x 106, or 10 x 106, depending on cell type; see Table 1). The injection was performed subcutaneously near the dorsal thigh region of the mouse.
For AB-ECM, the solution was thawed and kept at 4 °C throughout the protocol. The cell suspension (2X) was then mixed with AB-ECM at a 1:1 ratio (v/v). Again, the loading volume was 100 μL or 200 μL to obtain the desired number of cells; the number of cells for each cell type was equalized for the paired VitroGel and AB-ECM experiments. Following each round of inoculation, the syringe was put back on ice to prevent suspension solidification and needle clogging. Details of protocol components can be found in Table 1 below.
Table 1. Details of media, injection number, volume, and strain remained similar for use with both VitroGel and AB-ECM.
| Cell line | Culture media | Cell injection # | Injection volume | Mouse strain |
| PC3 | RPMI-1640, 10% FBS | 2 x 106 | 100 μL | Nude mouse |
| HeLa | EMEM, 10% FBS | 1 x 106 | 100 μL | Nude mouse |
| HCT-116 | McCoy’s 5A, 10% FBS | 10 x 106 | 200 μL | Nude mouse |
| HCC1806 | RPMI-1640, 10% FBS | 10 x 106 | 100 μL | Nude mouse |
| Calu-6 | EMEM, 10% FBS | 10 x 106 | 100 μL | Nude mouse |
| PANC1 | DMEM, 10% FBS | 10 x 106 | 200 μL | Nude mouse |
Tumor Tracking
Tumor size was measured two times per week using a caliper. Volumes were expressed in cubic millimeters using the formula: V (mm3) = (a (length) × b (width)2)/2, where a and b are the long and short diameters of the tumor, respectively. Averages and standard deviations among the (max = five; individuals were removed from the study upon >20% body weight loss, excessive stress, or death) mouse samples at each time point were calculated using Microsoft Excel. Experiments were continued until a predetermined tumor size (e.g., 1000 mm3) was reached. Animals were kept at room temperature (22 °C ± 3 °C) in 50% (±20%) humidity with a 12 h/12 h day/light cycle throughout the experiment. Appropriate IACUC approval was obtained prior to the initiation of the experiments, and ethical treatment of all animals was followed.
Results
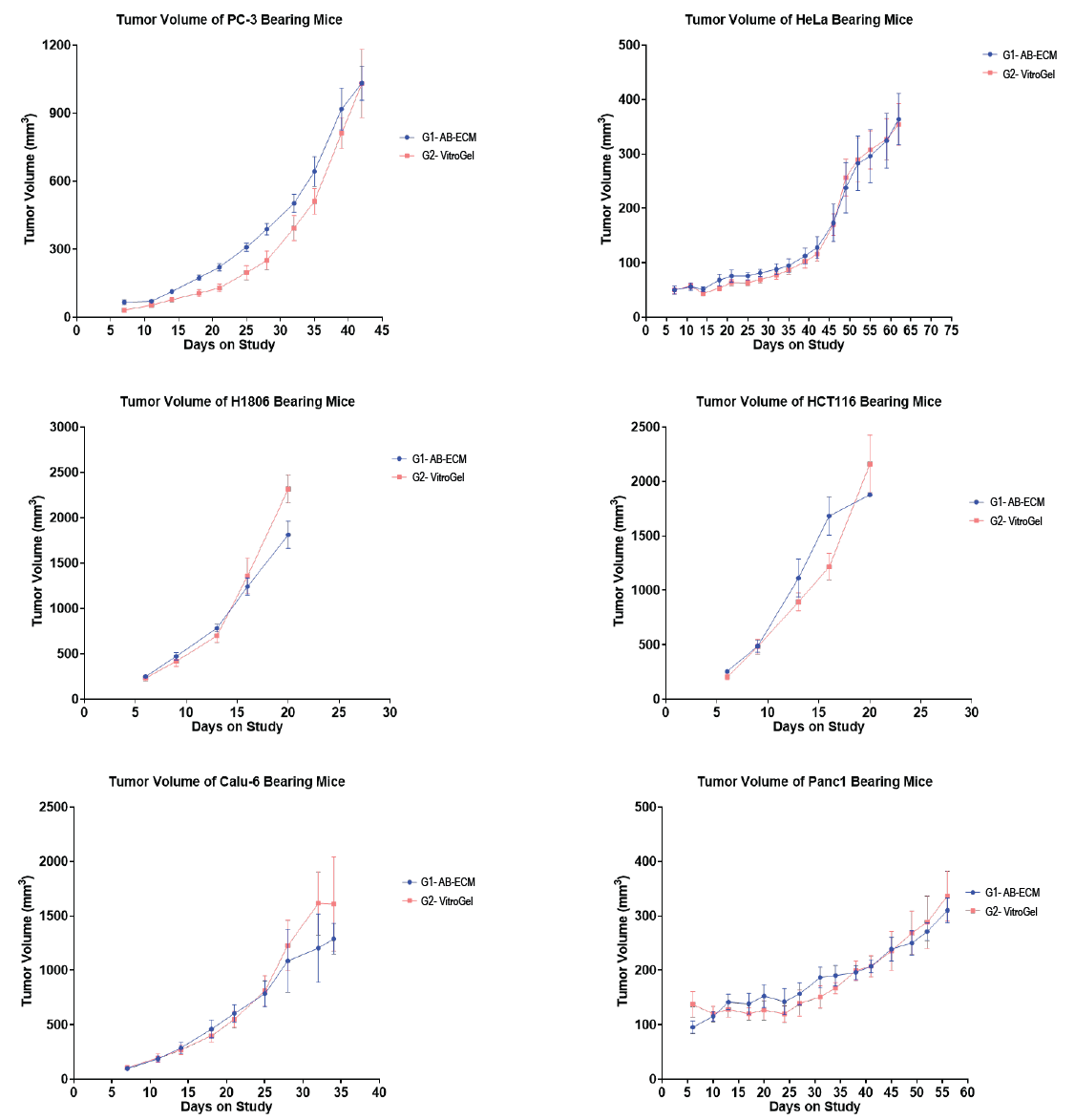
The observations were made over 2–10 weeks, depending on the tumor growth rate. A solid tumor was found to be forming successfully for all of the six solid tumor-derived cell lines when using either VitroGel or AB-ECM; tumor types and morphologies were analogous. The average growth rate was nearly similar in both AB-ECM and VitroGel for all cell types (Table 2; Figure 2). For all the six cell types, it was found that the time required to achieve the predetermined tumor size (Table 2) was the same in AB-ECM and VitroGel Hydrogel. Mice that showed a tumor growth size of greater than 2000 mm3 or those for which tumors exceeded 2 cm in any one dimension were neglected. Tumors grafted using VitroGel also displayed a lower standard deviation in most cases, implicating VitroGel as a reliable system that comes with less variability and more batch-to-batch consistency. These characteristics demonstrated the efficiency of VitroGel Hydrogel as a xeno-free substitute to AB-ECM, allowing it to be a promising alternative with the added ease of application. With superior properties like long-term injectable status, bulk sample preparation, and easy-to-use room temperature operation, VitroGel hence acts as a powerful tool for in vivo studies.
Table 2. Tumor growth endpoint times and tumor sizes for all six cell types in VitroGel.
| Cell type | Tumor growth endpoint | Final tumor volume (predetermined) |
| PC3 | 8 weeks | 1000 mm3 |
| HeLa | 7–8 weeks | 400 mm3 |
| HCT-116 | 2–3 weeks | 2000 mm3 |
| HCC1806 | 2–3 weeks | 2000 mm3 |
| Calu-6 | 4–5 weeks | 2000 mm3 |
| PANC1 | 8–9 weeks | 400 mm3 |
Discussion
The tumor growth curves, as seen above in Figure 2, indicate that there was successful solid tumor formation for all of the six cell types tested. The time required to reach a predetermined tumor size was comparable in both the AB-ECM and VitroGel, indicating the efficacy of VitroGel as a promising alternative to animal-based extracellular matrix for in vivo applications. Besides the six cell types above, VitroGel Hydrogel Matrix also supports many xenograft models and cell types: Breast (Hs578T, MDA-MB-231), Leukemia (CCRF-CEM, Reh), Lung (A549, H2170), Melanoma (A375), Pancreas (ASPC-1), Oral Cavity (HSC-2), Fibrosarcoma (HT1080), Renal (786-O), Tongue (CAL-27) and more.
Apart from the tumor growth success, coupled with no toxicity signs post-injection, VitroGel serves as a system that encompasses many practical benefits for cancer scientists. It helps encounter the challenge of maintaining a constant cold temperature of 4 °C during the pre-injection process, making its usage more flexible when ice is unavailable or inconvenient. A homogeneous cell suspension before injection, followed by a smooth injection administration, ensures reproducibility. With batch-to-batch consistency when compared to its AB-ECM counterpart, VitroGel is a reliable system for long-term analyses.
The system is intended to not only act as an alternative to AB-ECM but also to provide scientists the ability to control the supplements and growth factors they wish to add. This synthetic hydrogel system allows scientists to manipulate the key biophysical and biological properties such as mechanical strength, functional ligands, and degradability of the microenvironment. Hence it stands for a powerful biocompatible tool that lets one create many unique assays that native ECM can not do. Besides xenograft, the injectable VitroGel system is also excellent for other in vivo applications such as cell therapy, drug delivery, and wound healing.4-8
References
- Georges, L. M. C., De Wever, O., Galván, J. A., Dawson, H., Lugli, A., Demetter, P., & Zlobec, I. (2019). Cell Line Derived Xenograft Mouse Models Are a Suitable in vivo Model for Studying Tumor Budding in Colorectal Cancer. Frontiers in Medicine, 6. https://doi.org/10.3389/fmed.2019.00139.
- Dickinson, J., de Matas, M., Dickinson, P. A., & Mistry, H. B. (2021). Exploring a model-based analysis of patient derived xenograft studies in oncology drug development. PeerJ, 9, e10681. https://doi.org/10.7717/peerj.10681
- Xenograft | TheWell Bioscience. (n.d.). Thewellbio.com. Retrieved February 8, 2023, from https://www.thewellbio.com/applications/in-vivo/xenograft-pdx-cdx/
- Xenograft – In Vivo Injection, Xeno-free Hydrogel | TheWell Bioscience. (n.d.). Thewellbio.com. Retrieved February 8, 2023, from https://www.thewellbio.com/applications/in-vivo/tissue-engineering/
- Mori, H., Saeki, K., Chang, G., Wang, J., Wu, X., Hsu, P.-Y., Kanaya, N., Wang, X., Somlo, G., Nakamura, M., Bild, A., & Chen, S. (2021). Influence of Estrogen Treatment on ESR1+ and ESR1− Cells in ER+ Breast Cancer: Insights from Single-Cell Analysis of Patient-Derived Xenograft Models. Cancers, 13(24), 6375. https://doi.org/10.3390/cancers13246375
- Zhu, L., Feng, Z., Shu, X., Gao, Q., Wu, J., Du, Z., Li, R., Wang, L., Chen, N., Li, Y., Luo, M., & Wu, J. (2021). In situ transplantation of adipose-derived stem cells via photoactivation improves glucose metabolism in obese mice. Stem Cell Research & Therapy, 12(1). https://doi.org/10.1186/s13287-021-02494-4
- Xiao, M., Qiu, J., Kuang, R., Zhang, B., Wang, W., & Yu, Q. (2019). Synergistic effects of stromal cell-derived factor-1α and bone morphogenetic protein-2 treatment on odontogenic differentiation of human stem cells from apical papilla cultured in the VitroGel 3D system. Cell and Tissue Research, 378(2), 207–220. https://doi.org/10.1007/s00441-019-03045-3
- Wang, F., Nan, L., Zhou, S., Liu, Y., Wang, Z., Wang, J., Feng, X., & Zhang, L. (2019). Injectable Hydrogel Combined with Nucleus Pulposus-Derived Mesenchymal Stem Cells for the Treatment of Degenerative Intervertebral Disc in Rats. Stem Cells International, 2019, 1–17. https://doi.org/10.1155/2019/8496025
Links To Products/Services Used
All product names, logos, brands, trademarks and registered trademarks are property of their respective owners.