Application Notes
Advanced Skin Cell Models Using the VitroGel® System: 2D Coating and 3D Cell Culture with Human Dermal Fibroblasts
Application Note
Bhavya Shah, John Huang
TheWell Bioscience, North Brunswick, NJ 08902
Introduction
Despite recent progression, the need for reliable, repeatable in vitro three-dimensional (3D) tissue culture human skin models exist to further understand the mechanisms behind various pathologies related to the skin and/or dermal fibroblasts1-3. Such 3D models are increasingly important as two-dimensional (2D) human fibroblast cell culture systems lack the environmental factors (mechanical force, spatial orientation, nutrient and signaling impact) and cell-to-cell interactions that affect in vivo environments4. Likewise, the advanced 3D human skin models can provide new information on not only cell-to-cell interactions but cell-to-matrix and cell-to-environment communications, which are critical in skin disorders like Atopic Dermatitis, Psoriasis, and skin cancers5. Understanding how the extracellular matrix (ECM), nutrients, and other dermal components interact is important to understand these serious complications. The xeno-free functional VitroGel system utilizes tunable hydrogel properties to provide an excellent tool for establishing an in vitro 3D skin tissue culture model. Ultimately, this system allows scientists to manipulate in vitro skin models in a variety of ways to understand skin physiology and test topically applied products.
Here, we use the tunable VitroGel® RGD (TWG003) and the ready-to-use, multifunctional hydrogel VitroGel® Hydrogel Matrigel (VHM01). When used together, the tunable and hydrogel enable various cell culture models – in this case, normal human dermal fibroblasts (NHDFs) – to mimic in vivo systems such as the skin. Both the 2D hydrogel coating method and the 3D hydrogel encapsulation method were used, which allows for in-depth investigations of the dermal fibroblasts and their interactions with the surrounding environment. Below, our observations and analyses display the effects of mechanical strength of the hydrogel matrix, optimal seeding density, and the effects of collagen supplementation on the dermal fibroblast cells. Morphological properties of the fibroblast cell matrix, along with cell viability, were also evaluated via fluorescent microscopy and the Cyto3D™ Live-Dead Assay (BM01).
We hope this system serves as an inspiration for individuals to build complex tissue models that elevate drug screening processes, cell invasion assays, and disease-related research.
Materials and Methods
Cell Culture
Normal Human Dermal Fibroblast (NHDF) cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) with 10% Fetal Bovine Serum (FBS) and a 1x penicillin-streptomycin antibiotic. The cells were passaged when the cultures reached 80-90% confluence.
Adjustment of the Hydrogel Properties
To investigate the effects of the mechanical strength of the hydrogel on the growth of NHDF cells, the high concentration VitroGel RGD (TWG003, TheWell Bioscience, NJ) was used. The hydrogel solution was diluted with VitroGel Dilution Solution (Type 1) at both a 1:1 and 1:3 ratio (v/v) to test different final concentration hydrogel strength. The ready-to-use VitroGel Hydrogel Matrix (VHM01, TheWell Bioscience, NJ) was used to study the effect of supplementation of the NHDF cells in suspension. To test supplementation, cells were prepared in DMEM medium with or without FBS as a supplement. As a native ECM supplement, collagen was also added at concentrations of either 0.5mg/mL or 2.5mg/mL.
2D Hydrogel Coating Culture
Cells were harvested using trypsin and then resuspended in DMEM + 10% FBS at a seeding density of 0.5 x 106 cells/mL. The VitroGel RGD was diluted 1:1 or 1:3 and prepared for cell culture using the following steps:
- Prepare a cell culture medium (mixing medium) consisting of 50% FBS.
- Mix the diluted VitroGel RGD solution with the mixing medium from step 1 at a ratio of 4:1 (gel: medium, v/v). Gently mix the gel and medium to not introduce any bubbles to the solution.
- Add 50 µL of the hydrogel-cell medium mixture into each well of a 96-well plate.
- Let sit for 15-20 minutes at room temperature to allow for smooth gel formation and stabilization. Do not disturb the hydrogel by tilting or shaking the well plate, as this can disrupt the formation process.
- Carefully add 50 µL of the cell suspension to the top of the gel. Incubate at 37°C.
- Incubate the cells at 37°C and change 50-80% of the top medium every other day.
3D Culture
For the 3D culture of the NHDF cells, both the VitroGel RGD and VitroGel Hydrogel matrix were used. According to standard protocols, the mixing ratio of the hydrogel and the cell suspension is 4:1 (v/v) for the VitroGel RGD, and 2:1 (v/v) for the VitroGel Hydrogel Matrix. Therefore, for standard culture, the NHDF cells were placed in suspension with DMEM containing 50% FBS for the VitroGel RGD, and in DMEM medium with 30% FBS for the VitroGel Hydrogel Matrix. This results in a final FBS concentration of 10% for the hydrogel-cell mixture. For the 3D culture with VitroGel RGD, the cell suspension concentrations were 1-1.5 x 106 cells/mL. Cell suspensions for the 3D culture with VitroGel Hydrogel Matrix were prepared with or without FBS and collagen supplement as described above in the Adjustment of Hydrogel Properties section. The hydrogel-cell mixture was prepared using the following steps:
- Mix the hydrogel solution with the cell suspension at a 4:1 (v/v) ratio (VitroGel RGD) or at a 2:1 (v/v) ratio for the VitroGel HydroGel Matrix.
- Add 50 µL of the hydrogel-cell mixture into each well of a 96-well plate.
- Let sit for 15-20 minutes at room temperature to allow for smooth gel formation and stabilization. Do not disturb the hydrogel by tilting or shaking the well plate, as this can disrupt the formation process.
- Carefully add 50 µL of the cell suspension to the top of the gel. Incubate at 37°C.
- Incubate the cells at 37°C and change 50-80% of the top medium every other day.
Fluorescence Imaging
For fluorescence imaging, the cells were fixed and stained with ActinGreen™ and NucBlue™ to label F-actin filaments along with the cell nuclei. The following steps were used:
- Carefully, the cover media was removed from the top of the hydrogel with a pipette.
- The hydrogel was washed with 50 µL of D-PBS. The wash buffer was left to sit on the hydrogel for 3 minutes before removal.
- 50 µL of cell fixation solution (4% formaldehyde) was added to the surface of the hydrogel.
- The plate was incubated for 15-30 minutes at room temperature, followed by removal of the fixation solution.
- The hydrogel was gently washed three times with 50 µL of D-PBS, allowing the wash buffer to sit for 3 minutes between washes.
- 50 µL of permeabilization solution (0.1% Triton X-100) was added and incubated for 5 minutes at room temperature.
- The permeabilization solution was carefully removed and the well was washed three times with D-PBS.
- 50 µL of blocking solution (3% BSA in D-PBS) was added to the hydrogel and incubated for 60 minutes at room temperature.
- The blocking solution was removed, and ActinGreen™488 ReadyProbes™ reagent (ThermoFisher Scientific) was added according to their manufacture protocol.
- The plate was incubated for 30 minutes, followed by the addition of NucBlue™ Fixed Cell Stain Ready Probes™ reagent (ThermoFisher Scientific).
- The plate was incubated in the dark for 5 minutes, and fluorescence imaging was performed.
Live-Dead Assay
NHDF cell viability was monitored using the Cyto3D™ Live-Dead Assay Kit ( BM01, TheWell Bioscience, NJ) according to the following procedure.
- The Cyto3D Live-Dead Assay Kit was brought to room temperature.
- 2 µL of Cyto3D reagent was added to 100 µL total well volume or each well (50 µL hydrogel + 50 µL cover medium = 100 µL total volume).
- Cells were incubated at 37°C for 5-10 minutes and were then ready for cell viability detection using fluorescence microscopy.
- The cells were imaged using a fluorescence microscope. Live cells were observed using a GFP filter, while dead cells were observed using a Texas red filter.
Results and Discussion
MCF-7 cells were encapsulated in the hydrogel matrix for 3D cell culture. The cellular morphologies were monitored for 35 days. Figure 1 shows the 3D MCF-7 cell structures on days 1, 7, 14, and 35. Unlike the cell spheroid structure that is simply forming by aggregating cells into a round-shape colony in the U shape plate, handing drop plate or non-biologically functional hydrogel system, the functional VitroGel Hydrogel Matrix supports the formation of the luminal structure (red arrows in Figure 1) during cell spheroid formation since day 1. The luminal structure is an in vivo characterization of breast cancer spheroids, which plays a role in cancer progression. As the 3D spheroid structure grows, the luminal structures become more distinct at day 7 (Figure 1) when the cell clusters are about 50 µm in size.
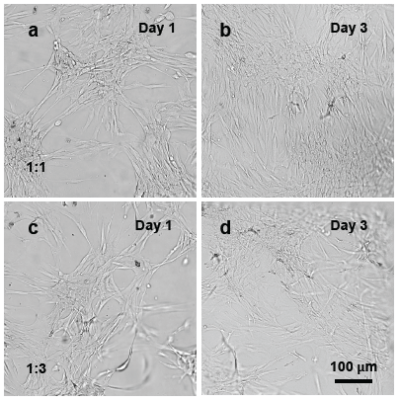
Figure 1. NHDF cells cultured on the surface of VitroGel RGD.
Vitrogel RGD with 1:1 (a,b) and 1:3 (c,d) dilution ratios were prepared using the 2D hydrogel coating. NHDF cells were added to the surface of the hydrogel. In both conditions, cells were able to attach to the hydrogel surface (Day 1) and spread for expansion (Day 3).
On the contrary, when NHDF cells were encapsulated within the VitroGel RGD for 3D culture, the mechanical strength of the hydrogel had a significant impact on human dermal fibroblast cell morphology. Figure 2 displays images of the NHDF cultured cells in the VitroGel RGD at both the 1:1 and 1:3 dilution ratios. The cells cultured in the 1:1 dilution ratio using the VitroGel RGD displayed a spheroid morphology and showed little, if any, cell-to-cell interactions or networking with neighboring cells. The cell proliferation was restricted by the strong mechanical strength of the hydrogel matrix. However, the softer 1:3 dilution ratio using the VitroGel RGD induced morphological changes to the fibroblasts on Day 1 after attachment to the gel. On day 7, these cells also continued to undergo morphological alterations and displayed clear cell-to-cell networking and cellular extension. These results suggest that the softer hydrogel at 1:3 dilution ratio confer a mechanically compliant environment to the cells, which mimics in vivo systems, as it allows the cells to undergo normal, morphological changes to interact with other cells and contribute to matrix mechanics6. These data suggest a clear advantage to the 3D hydrogel, specifically using the VitroGel RGD at a 1:3 ratio for studying human dermal fibroblasts, as the system better represents in vivo fibroblast physiology and cell networking1, 6.
Furthermore, when cellular extension occurs in the hydrogel matrix, the cell-cell interactions contribute to the cellular network formation. This formation is a direct indication of the cell seeding density on the 3D culture of NHDF cells. Therefore, we increased the concentration of cells within suspension from 1 x 106 cells/mL to 1.5 x 106 cells/mL. Figure 3 illustrates the phase contrast and fluorescence images of the growing NHDF cells, in the 1:3 diluted VitroGel RGD, for both the 3D culture and 2D hydrogel coating methods. Figure 3 depicts clear cell-cell interactions for those dermal fibroblasts grown on the 3D hydrogel matrix, starting as early as day 4. Fluorescence microscopy of the cells further demonstrates these cell-cell interactions as well as the development of a vast cellular network formation. This observation suggests a clear advantage for the utilization of the 3D hydrogel over 2D hydrogel coating systems as it allows for experiments that analyze such cell-cell interactions or network formation. Gaining a further understanding of the mechanisms related to these factors is important for our understanding of tumor formation and other diseases related to the skin and dermal cells, as the ECM undergoes vast amounts of remodeling during these complications7, 8.
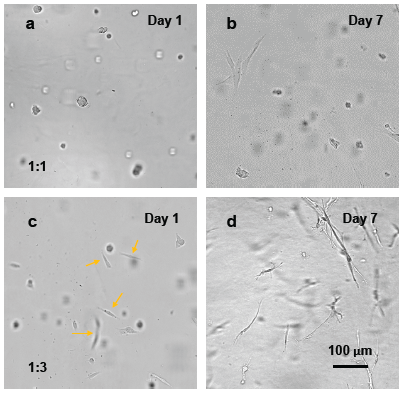
Figure 2. 3D cell culture of NHDF cells in VitroGel RGD.
VitroGel RGD at either 1:1 (a,b) or 1:3 (c,d) dilution ratios was used to mix with NHDF cell suspensions (1 x 106 cells/mL). In the softer, 1:3 diluted hydrogel, cells were more extended and undergo fibroblast morphology as indicated by the yellow arrows in day 1. After culturing cells in hydrogel for 7 days, the cells diluted at 1:3 ratio with the VitroGel RGD undergo clear fibroblast morphological changes and show cell-cell networking, while the cells diluted at a 1:1 ratio using the VitroGel RGD system maintain their spheroid structures with little cell extension.
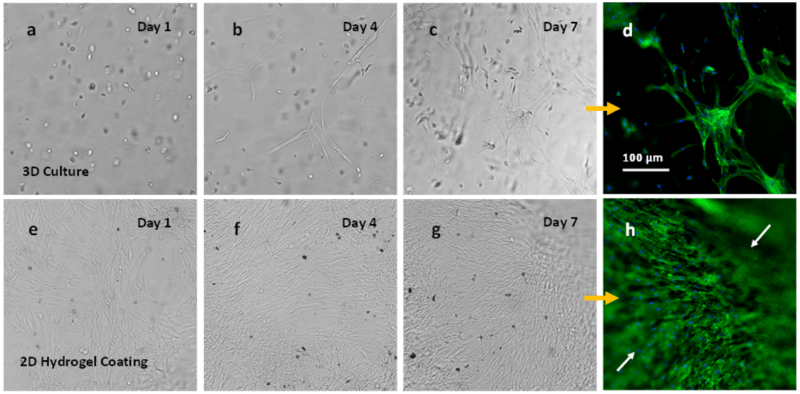
Figure 3. NHDF cells in VitroGel RGD (1:3 dilution) for both 3D culture and 2D hydrogel coating culture.
NHDF cells grow within the hydrogel matrix from day 1 to day 7 (Figures 3a-3c) at a cell suspension of 1.5 x 106 cells/mL in the hydrogel solution. Day 4 images (b) depict first evidence for cellular network formation in the 3D culture. Fluorescence images (d) illustrate cell-cell interaction with fibroblast cellular network structure formation for cells on the 3D culture. Images e-g display NHDF cells grown on the surface of VitroGel at a cell suspension of 0.5 x 106 cells/mL added to the top of the hydrogel). Cells attached to the hydrogel surface are shown since day 1 (e) until day 7 (g). Fluorescence images from the cells on the 2D hydrogel from day 7 are shown in Figure 3h and indicate cell invasion/migration into the hydrogel matrix (white arrows).
To study the effects of supplements on the 3D culture system with the NHDF cells, FBS and a native ECM agent (collagen) were used. The ready-to-use VitroGel Hydrogel Matrix was used to reduce the hydrogel preparing process and mainly focus on the medium supplementation. To evaluate the capability of the VitroGel Hydrogel Matrix to support the 3D cell culture of NHDF cells, a cell suspension with 30% FBS was used as a standard to mix with the hydrogel solution for 3D cell culture. Figure 5 displays the growth of the NHDF cells in the VitroGel Hydrogel Matrix. The images show clear NHDF cell proliferation over 7 days and morphological adaption beginning at day 4, indicating the 30% FBS media used in the cell suspension (which makes the final 10% FBS concentration in the hydrogel-cell mixture) is suitable as a standard. This 10% final concentration is equal to the standard 10% FBS media typically used for 2D culture of NHDF cells. Fluorescence microscopy revealed clear cellular network formation after 14 days of culture as well, providing further evidence for the suitability of the 30% FBS media.
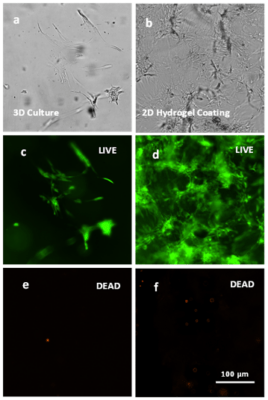
Figure 4. Live-Dead assay on NHDF cells on the VitroGel.
Live (green) and dead (orange) cells are depicted in both the 3D culture and 2D hydrogel coating culture one the VitroGel RGD at a 1:3 dilution using the Cyto3D Live-Dead Assay kit.
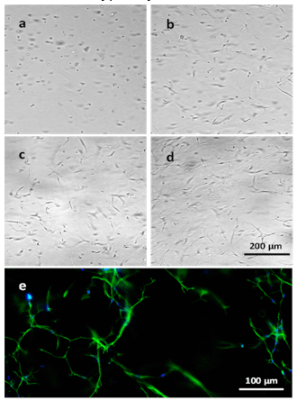
Figure 5. 3D culture of NHDF cells in VitroGel Hydrogel Matrix.
Images a-d display NHDF cells grown within the hydrogel matrix on days 0, 4, 7, and 14, respectively. Fluorescence image e displays cellular networking structure of cells cultured within the hydrogel over 14 days.
After showing optimal growth of the NHDF cells in the VitroGel Hydrogel Matrix with a 30% FBS supplement, we prepared cell suspensions with different supplement conditions to study the effects of medium supplementation on the 3D culture of NHDF cells. Figure 6 illustrates the result of the NHDF cells in 3D culture with or without FBS and collagen. Results from the experiment demonstrate that FBS plays a critical role in the 3D culture of NHDF cells, as it supports fibroblast morphology and promotes the formation of a cellular network structure with or without collagen supplementation. In any groups without FBS supplementation completely maintain their spheroid structure with almost no morphological changes over 4 days of culture on the VitroGel matrix. Cells without FBS supplementation also show little fibroblast extension over the 4 days. The one exception to this is that when cells are supplemented with the higher collagen dose (2.5mg/mL) without FBS, very little fibroblast extension does occur (Figure 6i, yellow arrows). These data suggest that high doses of collagen may aid in cellular network structure, but FBS is necessary to observe normal morphological changes and cellular network formations for the NHDF cells in 3D culture on the VitroGel Hydrogel Matrix.
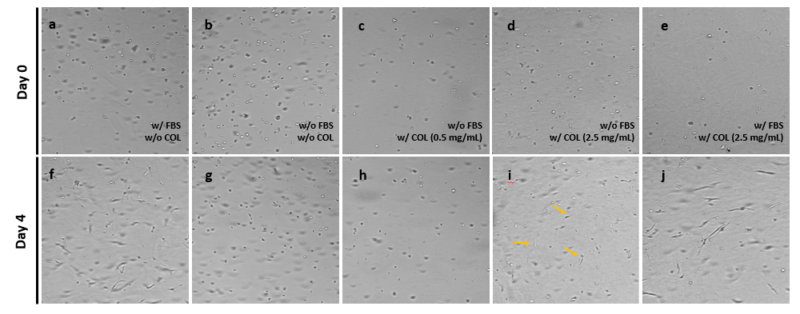
Figure 6. 3D culture of NHDF cells in the VitroGel Hydrogel Matrix with or without FBS and Collagen supplementation.
Images of the 3D culture of NHDF cells in the VitroGel Hydrogel Matrix with various supplemental conditions are shown on day 0 (a-e) and day 4 (f-j). Conditions were media with FBS but without Collagen (a,f), media without FBS and Collagen (b,g), media without FBS and with 0.5mg/mL Collagen (c,h), media without FBS and with 2.5mg/mL Collagen (d,i), and media with FBS and with 2.5mg/mL Collagen (e,j).
Concluding Remarks
The aforementioned data on the VitroGel system provide evidence for its use as a technical tool for the 3D culture of normal human dermal fibroblast cells. This tool can be utilized in a variety of methods, some of which we have demonstrated here where we exposed the fibroblasts to different supplemental conditions and assessed their growth, morphological changes, and ability to form cellular networks. Altogether, this robust 3D system represents a tunable tool that closely mimics in vivo human dermal fibroblast systems that encompass cell-to-cell interactions, cell-to-environment interactions and more. We hope this method provides easy access for investigators and researchers across the globe to build tissue models on this robust system for applications such as drug screening, invasion assays, and cancer research.
Reference
- Malakpour-Permlid, A., et al., Identification of extracellular matrix proteins secreted by human dermal fibroblasts cultured in 3D electrospun scaffolds. Scientific Reports, 2021. 11(1): p. 6655.
- Mesdom, P., et al., Human Dermal Fibroblast: A Promising Cellular Model to Study Biological Mechanisms of Major Depression and Antidepressant Drug Response. Curr Neuropharmacol, 2020. 18(4): p. 301-318.
- Szymański, Ł., et al., A Simple Method for the Production of Human Skin Equivalent in 3D, Multi-Cell Culture. International journal of molecular sciences, 2020. 21(13): p. 4644.
- Ali, N., et al., Skin equivalents: skin from reconstructions as models to study skin development and diseases. Br J Dermatol, 2015. 173(2): p. 391-403.
- Nyström, A. and L. Bruckner-Tuderman, Injury-and inflammation-driven skin fibrosis: the paradigm of epidermolysis bullosa. Matrix Biology, 2018. 68: p. 547-560.
- Rhee, S., Fibroblasts in three dimensional matrices: cell migration and matrix remodeling. Experimental & Molecular Medicine, 2009. 41(12): p. 858-865.
- Winkler, J., et al., Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nature Communications, 2020. 11(1): p. 5120.