White Papers
Patient-Derived Xenograft Using Xeno-Free VitroGel System in Comparison to Animal-based Matrigel
White Paper
The Jackson Laboratory, PDX R&D Core, Bar Harbor, ME
TheWell Bioscience, North Brunswick, NJ


Introduction
Patient-derived xenografts are becoming state-of-the-art for studying tumor behavior and drug responsiveness in a salient environment1. A patient-derived xenograft (PDX) is obtained when a tumor sample is removed from a patient, implanted into an immunosuppressed murine host, and then queried for cellular or molecular characteristics that could be of therapeutic value to the patient. The enthusiasm for the PDX approach is high because it allows not only for stable persistence of the tumor in vivo (in the mouse) but also because serial transplantation from one mouse host to others provides a tremendous opportunity to assay tumors over the long term. In this setting, PDX clinical trials (PCTs) address drug resistance issues that single-host scenarios cannot. They have also been exploited for archiving in communal repositories, such as those maintained by the National Cancer Institute (NCI) and the Jackson Laboratory, for multi-investigator use. Moreover, by its very nature, a PDX facilitates the study of variation between tumors of the same type and even heterogeneity within the same tumor.
Although the PDX technique has great promise and utility, some practical challenges remain to the xenograft procedure itself. Major such obstacles include labile cell preparation and graft rejection. Typically, the sample from the patient––and this could be derived from a biopsy, from primary tumor tissue, from metastatic tumor tissue, or from blood or lymph circulating tumor cells––is suspended in an animal-derived matrix (Matrigel®) to augment the actual injection procedure into the host. However, a fear of clumping, aggregation or other viscosity issues can cause the clinician to avoid keeping the suspension on ice, which mandates a short period between sample preparation and injection into the mouse. Plus, many non-xeno-free (non-animal-origin-free) hydrogels raise the specter that there will be an adverse reaction upon injection. If hydrogels for xenografting were free of these complications, clinicians would significantly enhance the efficacy of a PDX analysis.
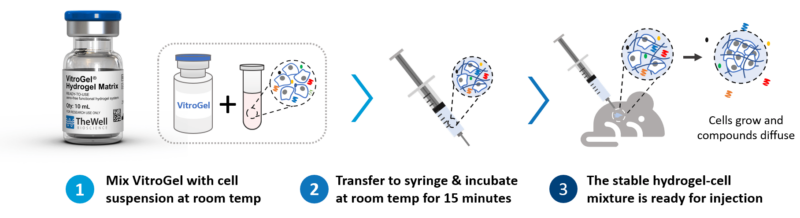
Thus, a comparison of a xeno-free hydrogel and an animal-based hydrogel for PDX was made here using a lung cancer cell system. In particular, the characteristics and ease of use of TheWell Bioscience’s VitroGel® Hydrogel Matrix (SKU: VHM01), was compared side-by-side with Matrigel and a pure saline solution for the xenograft application in a PDX context. See Figure 1 for the protocol overview.
In this study, NSGTM mice were xenografted with a PDX model using either Matrigel, VitroGel, or saline as the injectable carrier medium. The PDX model chosen was TM00244, a primary malignant stage 2 lung squamous cell carcinoma from a 62-year-old Caucasian female. The results show that VitroGel Hydrogel Matrix can support a higher tumor formation rate and fast cell growth kinetics compared to Matrigel.
Materials and Methods
NSGTM scid mice with genotype NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ were xenografted with PDX model TM00244 cells suspended with Matrigel, VitroGel, or saline. Tumor fragments of 1cm3 were ground with mortar and pestle in DPBS. The cells were further dispersed by a 19G needle and syringe. The volume of resuspension was 500 μL. Then, 200 μL of the carrier gel (or saline) was mixed with 100 μL of the tumor suspension at room temperature. After 15 minutes, 50 μL was injected into mice (N = 3 per treatment).
Tumors were measured by the standard caliper methodology each week for 8 weeks. Tumor sizes were analyzed by one-way ANOVA with multiple comparisons for statistical significance among the treatment regimens (carrier gels).
Results and Discussion
Injection of cells suspended in VitroGel proceeded smoothly. Observation following injection on the same day was that the “tumor only” (saline) and “tumor and Matrigel” mice appeared to have darkening and bruising at the injection site. By contrast, mice injected with “tumor and VitroGel” did not display any darkening or bruising at the injection sites.
Importantly, mice injected with VitroGel sustained tumor growth exceptionally well over the 8-week duration of the experiment (Figure 2A). Growth accelerated after 3 weeks and reached a peak size at 6 weeks. At the 8-week time point, the tumor sizes from VitroGel-supported injections were significantly larger than either those supported in Matrigel or saline solution alone (Figure 2B). There was no significant difference in tumor growth when the Matrigel was compared with the saline solution.
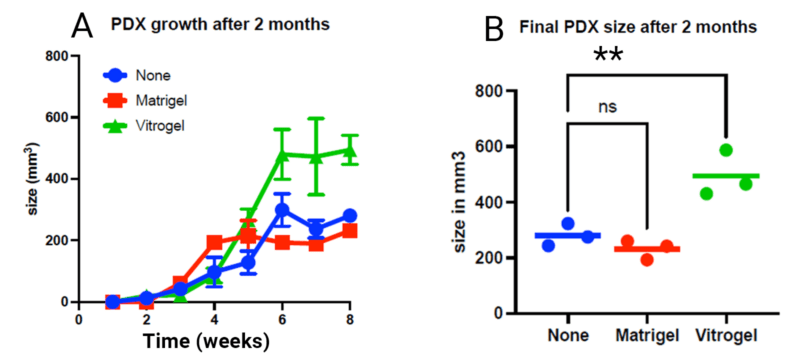
Overall, it can be concluded that an injection supported with VitroGel is clearly superior to Matrigel or saline solution in terms of consistency and ease of use. VitroGel outperforms Matrigel as measured by the maintenance of tumor growth in a lung cancer PDX sample repeated in triplicate. Matrigel is animal-based, containing about 2,000 undefined compounds. The system has known batch-to-batch inconsistency and is thus less able to support clinical applications and is challenging to use for high-throughput and lab automation. VitroGel has none of these problems. It is also stable for an extended period of time after mixing with a cellular sample, obviating the need to rush samples from patients to the PDX host. Tumors that grew in the host following injection aided by VitroGel displayed robust long-term growth significantly better than that seen with Matrigel.
More About VitroGel
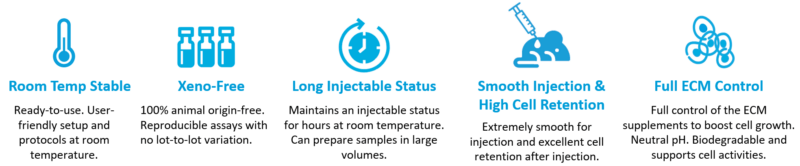
TheWell Bioscience’s VitroGel is a superior alternative to animal-based and plant-based ECM as an injectable carrier for xenograft, targeted delivery, controlled release, and cell therapy for tissue engineering. As an efficient hydrogel system, VitroGel carries cells/tissue into the target injection site, maintains excellent cell retention at the injection location, and supports cell growth after delivery. Unlike animal-based ECM, which requires delicate cold temperature operation, VitroGel is an easy-to-use hydrogel solution with the operation setup and cells/compounds hydrogel mixture at room temperature, see figure 3. After a simple, quick, and easy preparation setup, it is ready for injection and maintains injectable status for hours at room temperature or 37° C.
Because VitroGel has a unique rheological property that can maintain an excellent injectable status (reversible sol-gel transition status) for hours after mixing with cells, scientists can prepare samples in bulk and continue to inject without worrying about needle clogging. The xeno-free bio-functional hydrogel system has many other advantages (figure 3). During animal safety studies, VitroGel demonstrated biocompatibility without showing a toxic or inflammatory response. Researchers have full control over the ECM supplements to optimize and boost cell growth. In addition, VitroGel is not constrained by supply chain issues and is manufactured with batch-to-batch consistency for reproducible lots.
References
- Risbridger, P., Lawrence, M. G., & Taylor, R. A. (2020). PDX: Moving Beyond Drug Screening to Versatile Models for Research Discovery. Journal of the Endocrine Society 4(11). https://doi.org/10.1210/jendso/bvaa132
- Pang K., Park J., Ahn S.G., et al. (2019). RNF208, an estrogen-inducible E3 ligase, targets soluble Vimentin to suppress metastasis in triple-negative breast cancers. Nat. Comm. 10: 5805 doi:10.1038/s41467-019-13852-5.
Links To Products Used
All product names, logos, brands, trademarks and registered trademarks are property of their respective owners.