Application Notes
Xeno-free Organoid Generation Workflow for Stem Cell Spheroids Using VitroGel® STEM and VitroGel® ORGANOID Hydrogel System
Application Note
TheWell Bioscience, North Brunswick, NJ 08902
Over the past decade, advances in stem cells and 3D cell culture technologies have reignited interest in organoid research. Organoids are derived from stem cells such as human pluripotent stem cells (hPSCs) or stem/progenitor cells and are composed of different cell types that are able to self-organize, forming a 3D structure that is meant to be a miniature mimic of organs1. Studies have explored and highlighted the tremendous potential of organoids in modeling complex human diseases, understanding human development, and in the field of regenerative medicine. Today, different organoids resembling different human organs can be made in the lab – brain, retinal, stomach, esophagus, lung, small intestine, liver, pancreas, kidneys, blood vessels, etc2. The list will continue to grow as more research efforts are channeled towards organoid generation.
Current organoid models heavily rely on animal-derived extracellular matrix (ECM) such as Matrigel. However, the components of Matrigel are undefined, and there are variations between batches. The inconsistencies of such an ECM substrate greatly limit the reproducibility of current organoid models and are unsuitable for clinical applications. Additionally, traditional organoid culture methods typically start from hPSC monolayer cultures, where monolayer hPSCs are harvested as single cells or small cell clusters and aggregated in 3D cultures. At present, there are several critical limitations of current organoid culture methods: Firstly, animal-derived matrices are unstable and unsuitable for clinical applications. Secondly, recovering hPSCs from frozen stocks using conventional 2D monolayer culture methods often results in loss of cell viability and is a time-consuming process. Next, dissociating hPSC colonies as single cells is a harsh technique that will dramatically reduce cell viability3, therefore compromising the quality of the hPSC aggregates formed. Lastly, this traditional method has limited scalability. This is a major barrier to large-scale, 3D-based production of both hPSC and organoids, which will be important for organoids to be used as clinical therapeutics moving forward. Therefore, there is an imperative need for a method that allows for faster and easier organoid differentiation that is highly scalable while still maintaining the high quality of cells.
This application note described a method to generate and culture mid/hind-gut and organoids from hPSCs using the 3D xeno-free hydrogel systems – VitroGel® STEM and VitroGel® ORGANOID (Figure 1).
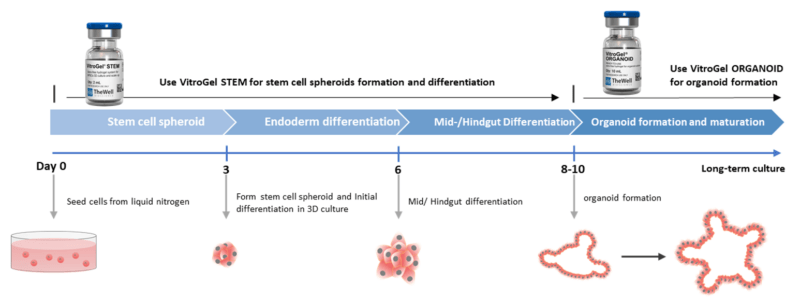
These systems allow for a quick and easy generation of 3D hPSC spheroids from monolayer hPSCs or even directly from frozen hPSC stocks just by mixing these cells with VitroGel® STEM (Figure 2). Within three days, hPSC spheroids are formed, which can be differentiated into mid/hind-gut with the same hydrogel 3D culture system and further be used to generate organoids with VitroGel ORGANOID.
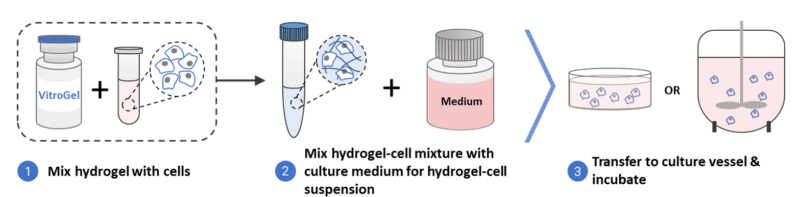
Both VitroGel® STEM and VitroGel® ORGANOID are xeno-free, providing robust cell expansion and differentiation processes, and the organoids generated from this method open doors to downstream clinical applications. Our VitroGel® substrates can overcome the drawbacks of current ECM for stem cell cultures and organoid generation – firstly, our xenofree substrates are stable and suited for downstream clinical applications. Secondly, VitroGel® STEM is able to improve cell viability after hPSCs are thawed from frozen vials. Additionally, our hydrogel-based substrates are highly scalable, allowing large-scale expansion of stem cells and even organoid production. Hence, VitroGel® substrates can enhance the quality and reproducibility of organoids generated, as well as the viability of stem cell cultures.
Materials and Methods
Forming 3D spheroid cultures from frozen vials or 2D cultures of hPSCs
VitroGel® STEM is able to support the recovery and expansion of thawed hPSCs to form 3D hPSC spheroids (Figure 3). Conventional methods require the culturing of hPSCs on a matrix for multiple passages before hPSC cell lines recover and stabilize. During the thawing process, preserving the viability of thawed hPSCs is typically challenging. However, VitroGel® STEM eliminates that difficulty by allowing 3D spheroids to be generated from cells taken out from liquid nitrogen. Shaking or rocking is not needed for 3D spheroid formation.
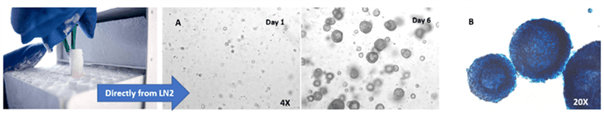
A. hPSC spheroids formed directly from cells stored in liquid nitrogen grow continuously from day 1 to 6. B. Alkaline Phosphatase (AP) staining shows that these spheroids are healthy and that they retain their pluripotency indicated by high levels of AP expression.
Generating 3D spheroids by the following protocols:
- Bring VitroGel® STEM to room temperature or warm up to 37°C.
- Resuspend thawed cells in the appropriate media before centrifuging to obtain cell pellet and remove cell freezing solution.
- Prepare cell suspension in stem cell media with 10μm/mL ROCK Inhibitor Y-27632 *.
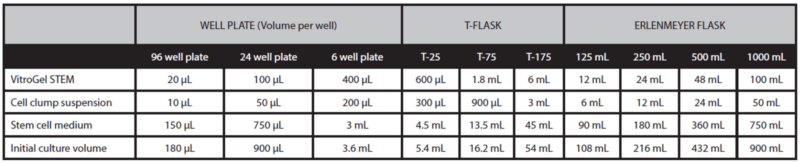
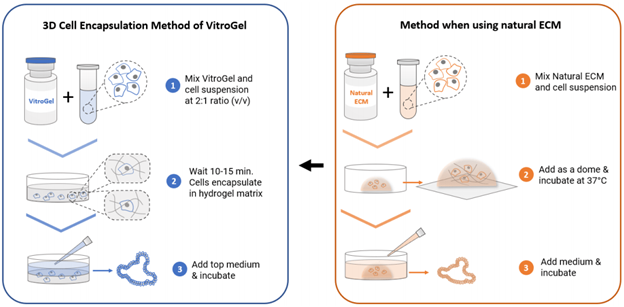
- Gently mix VitroGel® STEM with cell suspension at 2:1 (v/v) ratio. (Check Table 1 or Table 2 of the recommended volume of different culture vessels for different culture cycles)
- Add stem cell medium with 10μm/mL Y-27632 to the cell‐hydrogel mixture at a 5:1 (v/v) ratio. This ratio is critical in ensuring hPSC spheroids are well-formed. Carefully pipette up and down to mix the medium and mixture homogeneously**.
- Add the desired volume of the mixture to the culture vessel and incubate at 37°C with 5% CO2 (See Tables 1 and 2).
- Add additional medium for 7‐day culture cycle: On day 3 or day 4, add the desired volume of stem cell medium (without Y‐27632) directly to the culture vessel (See “additional medium” volume in Table 1).
* The recommended cell density is 0.2‐5 X 106 cells/mL for the final cell seeding density in the hydrogel suspension culture around 0.1‐5 X 105 cells/mL. The cell seeding density should be optimized for individual cell types and will also depend on the desired final sizes of the stem cell spheroid.
** The mixing ratios of stem cell medium and cell‐hydrogel mixture can be adjusted between 1:1 to 20:1 v/v ratio, depending on the desired viscosity of the final mixture and culture conditions. If the mixing ratio is higher than 5:1 v/v, an orbital shaker or spin flask may be required and set at a speed of 10‐40 rpm to maintain the cell suspension.
Note: 3D hPSC spheroids can also be made from hPSCs cultured in conventional 2D monolayers. If monolayer hPSC cultures are used, dissociate cells using a suitable dissociation reagent (as per manufacturer’s recommendations) and then continue from steps 3 onwards.
Table 1. Recommend volume for 7-day culture cycle
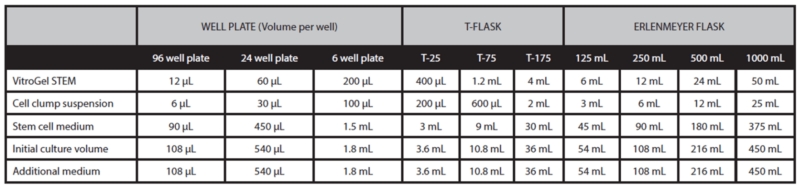
Table 2. Recommend volume for 3-day or 4-day culture cycle
3D endoderm and mid/hind-gut differentiation with VitroGel® STEM
The hPSC spheroids were then collected and directed to differentiate into the endoderm lineage by:
- Transfer hPSC spheroids from the culture to a conical tube by using a serological pipette.
- Centrifuge the tube for 3 minutes at 100 x g.
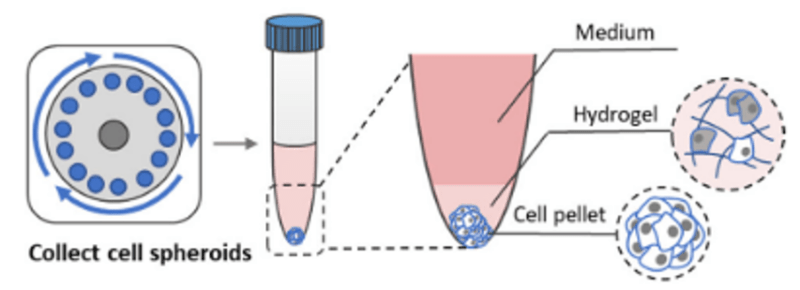
- Carefully remove supernatant and hydrogel layer and collect cell pellet (Figure 4).
Note: Alternatively, a modified enzyme-free cell recovery method using VitroGel® Cell Recovery Solution can be utilized* (check the protocol of Cell harvesting using VitroGel® Cell Recovery solution below).
- Resuspend hPSC spheroids with endoderm differentiation medium.
- Mix VitroGel® STEM with spheroid suspension at 2:1 (v/v) ratio.
- Mix the additional endoderm differential medium with the cell-hydrogel mixture from step 5 at 5:1 (v/v) ratio for suspension culture for 3 days. (Check Table 2 of the recommended volume of different culture vessels)
- After 3 days, harvest the endoderm spheroids by repeating steps 1-3.
- Prepare endoderm spheroids suspension in mid/hind-gut differentiation medium
- Mix VitroGel® STEM with spheroid suspension at 2:1 (v/v) ratio.
- Mix with additional mid/hind-gut differential medium with the cell-hydrogel mixture from step 9 at 5:1 (v/v) ratio for suspension culture for 2-4 days. (Check Table 2 of the recommended volume of different culture vessels)
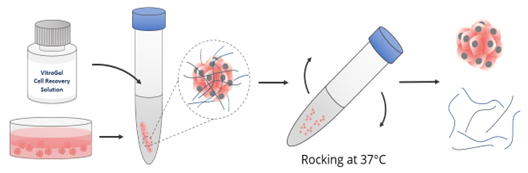
*Cell harvesting using VitroGel® Cell Recovery Solution
- Add warm VitroGel® Cell Recovery Solution (2-5X volume) directly to spheroids in the culture plate or a tube (Figure 5).
- Mix gently by rocking or shaking the plate/ pipetting up and down 3-5 times.
- Incubate the mixture at 37°C for 3-5 minutes.
- Transfer the mixture to the centrifuge tube and centrifuge at 100 x g for 3-5 minutes
- Carefully remove supernatant and hydrogel layer and collect cell pellet (Figure 4).
Organoid formation and maturation
The mid/hind-gut spheroids were then collected, and organoids were formed by:
- Transfer mid/hind-gut spheroids from the culture to a conical tube by using a serological pipette.
- Centrifuge the tube for 3 minutes at 100 x g.
- Carefully remove supernatant and hydrogel layer and collect cell pellet (Figure 4).
- Bring VitroGel® ORGANOID hydrogel to room temperature or warm at 37°C.
- Add 1 mL VitroGel® ORGANOID to 500 μL cell suspension and carefully pipette up and down 5‐10 times to mix thoroughly (Keep VitroGel® and cell suspension at 2:1 v/v mixing ratio). Note: If critical growth factors such as R-spondin, Noggin or EGF are needed in the hydrogel to support the organoid culture, prepare the cell suspension with 3x critical growth factors (Please check the user protocol of VitroGel® ORGANOID for details).
- Transfer the hydrogel mixture to a well plate. Gently tilt/swirl the well plate to ensure even coverage on the bottom of each well.
The recommended volume of hydrogel for specific well plates is listed below.
| 6 well plate | 12 well plate | 24 well plate | 48 well plate | 96 well plate | |
| Volume per well | 1200ul | 600ul | 300ul | 150ul | 50ul |
- Let mixture stabilize at room temperature for 10‐15 min.
- Carefully cover the hydrogel with additional medium.
- The recommended volume of hydrogel for specific well plates is listed below:
| 6 well plate | 12 well plate | 24 well plate | 48 well plate | 96 well plate | |
| Volume per well | 1200ul | 600ul | 300ul | 150ul | 50ul |
- Place the well plate in an incubator and change the top medium according to the experiment’s design.
Note: Change 50‐80% of the top medium with the same type of culture medium used. Change 100% of the top medium if switching to a different medium during organoid culture. Avoid disturbing the hydrogel during medium changes.
Results and Discussion
VitroGel® STEM maintains pluripotency of hPSCs
One of the key concerns when it comes to culturing hPSCs is unwanted spontaneous differentiation resulting in the loss of pluripotency. This will negatively impact downstream applications, including gene editing, tissue organoid development, cell reprogramming, biochemical assays, and DNA/RNA sequencing due to the reduced purity of differentiated cell populations. This has especially significant implications in stem cell-based therapies where the purity of differentiated cells is crucial in restoring tissue functions. Immunofluorescence staining on the 3D hPSC spheroids cultured in VitroGel® STEM for 3 days shows that the 3D hPSC spheroids retain high expression levels of pluripotency markers SSEA, OCT4, SOX2, and TRA-1-60 (Figure 7).
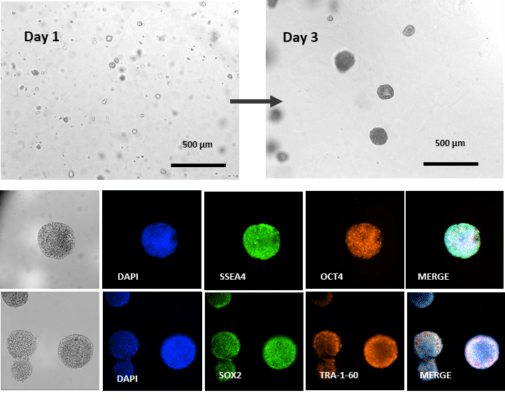
Differentiation of hPSC spheroids to mid/hind-gut organoids
The hPSC spheroids were easily differentiated into the endoderm lineage. On day 3, the hPSC spheroids were harvested and subcultured in VitroGel® STEM with the endoderm differentiation medium. By day 6, endoderm spheroids of about 200uM were generated (Figure 8). Endoderm spheroids appear dense and spherical. The endoderm spheroids were then harvested and sub-culture in VitroGel® ORGANOID with the mid/hind-gut differentiation medium. One day after mid/hind-gut differentiation, the gross morphology of the organoids changed drastically (Figure 8, Day 7); the surface of the organoids appeared to be more ‘irregular,’ indicating cell ‘budding,’ which is a common characteristic of epithelial tissues4. By day 8, more epithelial ‘buds’ are formed. Immunofluorescence imaging confirmed that these organoids express key epithelial markers E-Cadherin and mid/hind-gut marker CDX2 (Figure 8). Here we show that this innovative hydrogel-based method yielded high-quality hPSC spheroids as well as organoids. The size of the spheroids and organoids is uniform and consistent. In addition, the hydrogel substrate supported further differentiation and maturation of mid/hind-gut organoids. Therefore, the results illustrate the utility of VitroGel® STEM and VitroGel® ORGANOID in achieving successful organoid production.
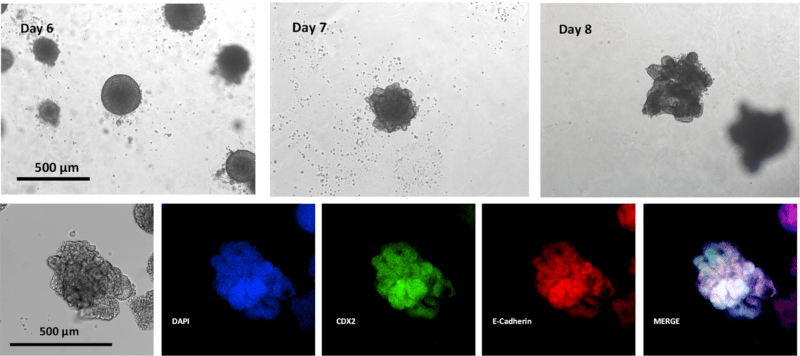
VitroGel® ORGANOID permits long-term culture and maturation of mid/hind-gut organoids
After confirming the formation of mid/hind-gut at day 9, we harvested the cells and mixed the mid/hind-gut with VitroGel ORGANOID for organoid formation and maturation (Figure 9). VitroGel® ORGANOID is able to promote long-term culture and mid/hind-gut organoids. By day 16, mid/hind-gut organoids that were encapsulated in VitroGel® ORGANOID further developed and matured. The organoids increased in size and developed significantly more epithelial buds, which is indicative of further organoid growth and maturation.
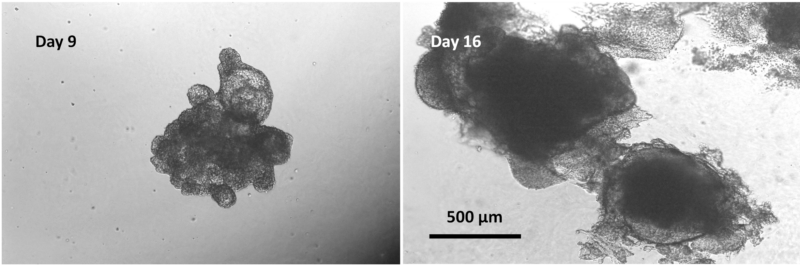
In summary, this study illustrates VitroGel® STEM, a xeno-free hydrogel that can be used to culture and maintain hPSCs as 3D spheroids. It can even promote hPSC cell recovery after immediately thawing from frozen vials and forming spheroids. This essentially eliminates 2D hPSC monolayer cultures, which may negatively influence the viability of hPSCs and, later, organoids. 3D hPSC spheroids formed using that VitroGel® STEM express high levels of key pluripotency markers, which illustrates that the method maintains the high quality of hPSCs. These hPSC spheroids can then be easily directed to differentiate into organoids such as the mid/hind-gut organoids. VitroGel® ORGANOID is also able to effectively promote further organoid growth and development, allowing for long-term, high-quality cultures of these organoids. We show that VitroGel® STEM and VitroGel® ORGANOID effectively streamline the organoid differentiation process, increasing the ease of generating organoids for a variety of applications. Organoids produced as uniform and consistent, overcoming issues pertaining to reproducibility and increasing success rates of downstream assays.
References
- Clevers, H. Modeling Development and Disease with Organoids. Cell 165, 1586–1597 (2016).
- Kim, J., Koo, B.-K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol 21, 571–584 (2020).
- Chen, K. G., Mallon, B. S., McKay, R. D. G. & Robey, P. G. Human Pluripotent Stem Cell Culture: Considerations for Maintenance, Expansion, and Therapeutics. Cell Stem Cell 14, 13–26 (2014).
- Wang, S., Matsumoto, K., Lish, S. R., Cartagena-Rivera, A. X. & Yamada, K. M. Budding epithelial morphogenesis driven by cell-matrix versus cell-cell adhesion. Cell 184, 3702-3716.e30 (2021).