2D Hydrogel Coating
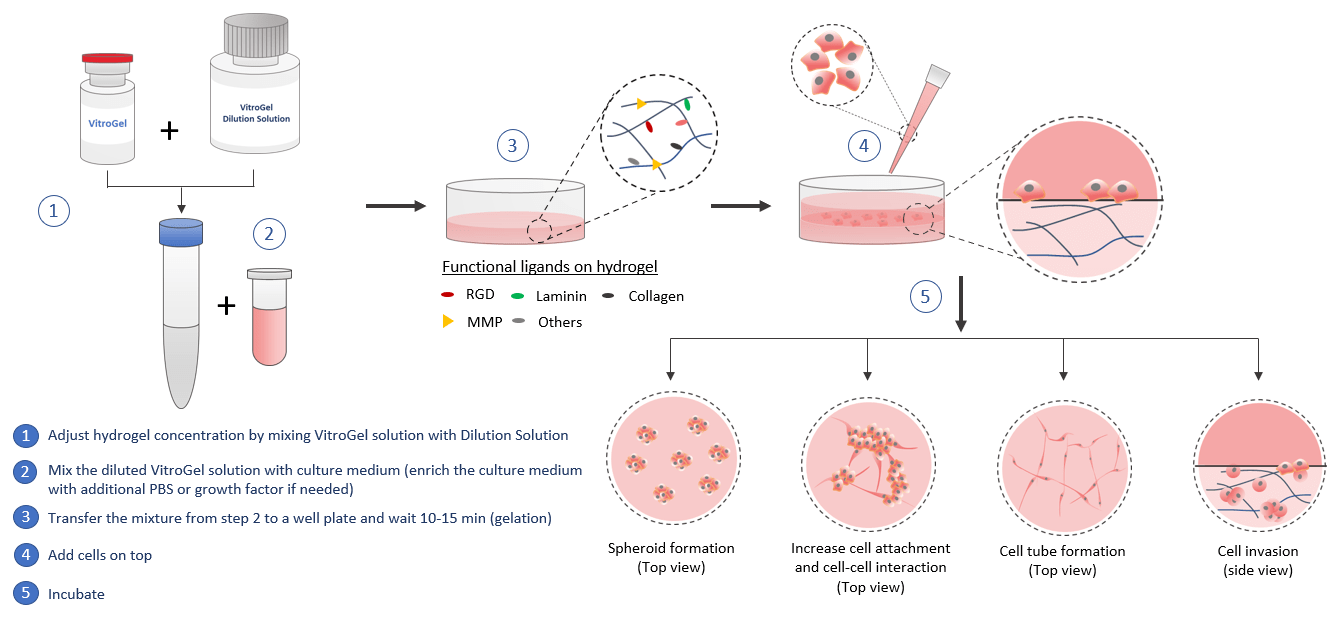
VitroGel® can be used for 2D hydrogel coating. By adjusting the hydrogel concentration and selecting different biological functional ligand modified hydrogel, scientists can study the cell responses to different substance stiffness and cell-matrix interactions. The quick cell spheroid formation can happen when cells migrate/aggregate on the surface of the hydrogel. With the soft substance, cells can submerge into the hydrogel for a 2D to 3D cell culture which is a great method to bridge the understanding of 2D cell culture and 3D encapsulated cell culture. The functional ligands on the hydrogel can increase the cell attachment and promote the cell-matrix and cell-cell interactions including the growth factors that induced cell tube formation. The 2D coating VitroGel can also be a powerful system to study cell invasion. Drug screening, immunofluorescence analysis, and cytotoxicity assays can perform in hydrogel directly. Cells cultured in this system can be easily harvested out with VitroGel Cell Recovery Solution for downstream analysis or subculture.
Similar to the 3D cell model, by using the VitroGel for 2D hydrogel coating application, scientists can get full control of the biomatrix microenvironment by manipulating the biophysical and biological properties of the hydrogel.
Mechanical strength
In VitroGel, the hydrogel strength can adjust by a simple dilution process. The typical gel strength range from 10 to 4,000 pa and the customized higher concentration hydrogel can get over 20,000 Pa.
Degradation
The MMP modified VitroGel can be used for this study. The hydrogel can mix and match with other versions of VitroGel to study the process of MMP degradation and cell-matrix interaction for cell mobility.
Functional Ligands
With VitroGel, scientists can select hydrogel with different functional adhesive ligands, such as RGD, collagen, laminin, and combine/vary the concentration to form a heterogeneous customized microenvironment.
Serum/growth factors/cytokine/chemokine
VitroGel is the xeno-free system. Bio-functional compounds can add to cell suspension before mixing with hydrogel to create an enriched hydrogel matrix or add after the hydrogel formation to feed cells.
Spheroid Formation
![]()
Spheroid Formation
By adjusting the stiffness and the surface property of the substance, hydrogel 2D coating can support the cell assembly. The cell spheroid formation can happen when cells migrate/aggregate on the surface of the hydrogel.
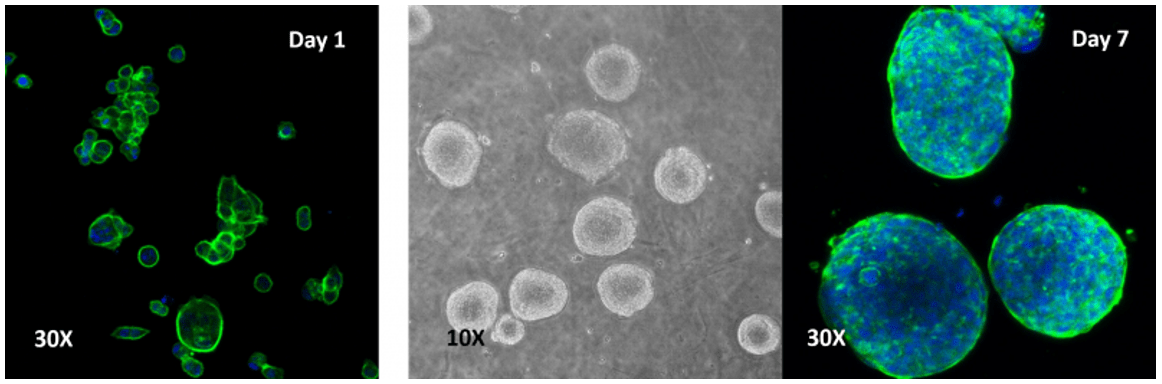
Human colon cancer cells (HCT 116) cells cultured on top of VitroGel 3D hydrogel.
A thick hydrogel coating plate has been prepared by mixing VitroGel 3D with PBS at 1:1 ratio. A 300 µL mixture has been added to a well of a 24-well plate and stabilization at room temperature for 20 minutes before adding cells on top of the hydrogel. Cells acquired a spheroid shape after 24 hours and continued to increase in size out to 7 days.
Human Lymphoblastoid Priess cells cultured on top of VitroGel 3D hydrogel
A. Priess cells were grown in suspension (control); B. Priess cells grown on top of VitroGel 3D at day 7. Cells seeded on the top of the hydrogel form cell spheroids form on the top of the hydrogel. The hydrogel provides a soft substance for cells to attach and grow.
Increase cell attachment and cell-cell interaction
![]()
Increase cell attachment and cell-cell interaction
The adhesive ligands on functional VitroGel can support the cell attachment, which can further improve cell-cell interactions. The 2D hydrogel coating provides an alternative model to bridge the traditional 2D well-plate culture and the 3D cell encapsulating culture.
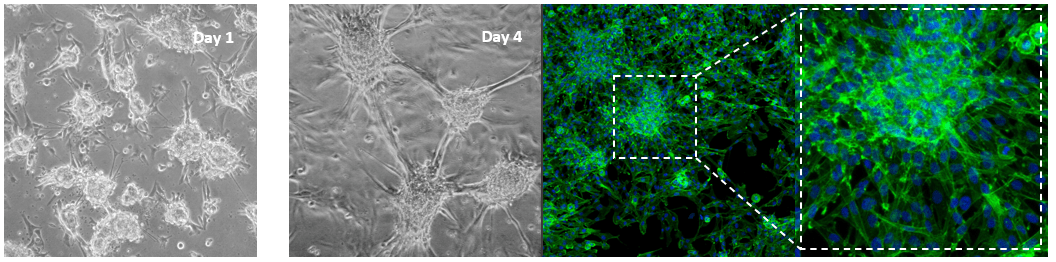
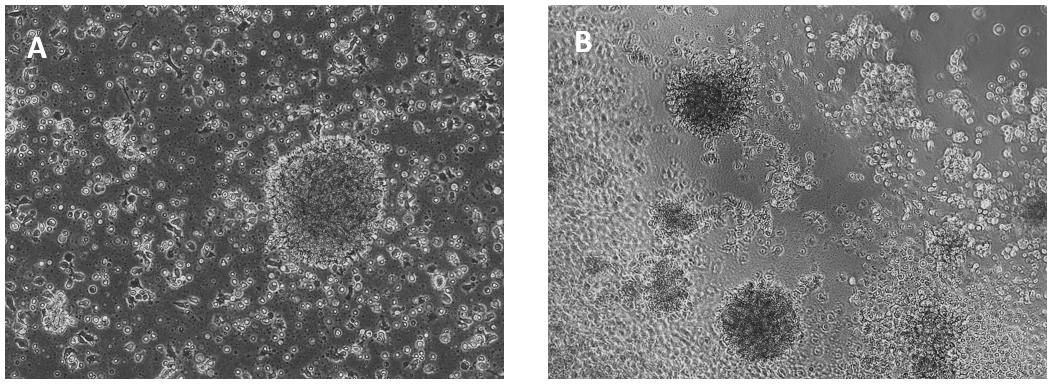
Glioblastoma cells (SNB 75) culture on 2D VitroGel RGD coating surface
The hydrogel coating surface was prepared by mixing VitroGel RGD with cell culture medium at 4:1 (v/v) ratio. The gelation process was stable at room temperature for 20 min before adding cells on top. Cells attached and formed the colony structure on the surface of the hydrogel (day 1). The attached cells expanded and connected colonies for the next working structure (day 4).
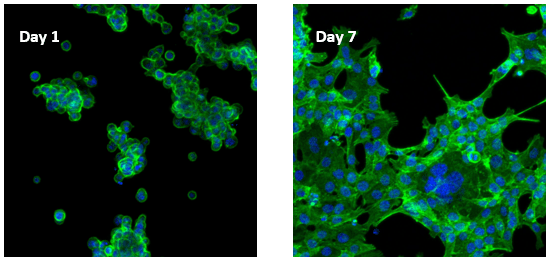
Human colon cancer cells (HCT 116) cells cultured on top of VitroGel RGD hydrogel
Cells on VitroGel 3D-RGD began to aggregate and form spheroid cell clusters after 24 hours. The aggregated cells then spread out on the surface of the hydrogel and connected each other (day 7).
Cell Tube FoRMATION
![]()
Cell Tube Formation
Endothelial cell can growth on the surface of VitroGel. With additional growth factors supplement, the hydrogel can support the cell tube structure formation. Scientist can get full control by selecting the functional hydrogels and adjusting the concentrations of hydrogel and growth factors to get the optimal conditions for different applications.
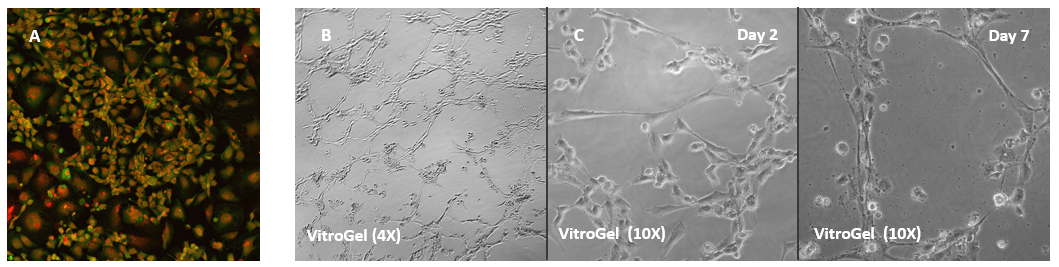
Human Umbilical Vein Endothelial Cells (HUVEC) cultured on VitroGel 2D coating surface
A. HUVEC cells culture on the surface of the VitroGel Angiogenesis Assay Kit without additional growth factor supplement. The cells can attach and spread on the surface of VitroGel. B. When the hydrogel was supplement with growth factors and cells were culture in the medium with growth factors, the tube structure can form on the surface of VitroGel 24 hours after cell seeding. C. Cells cultured on the surface of VitroGe with a low concentration of growth factor supplement can slowly induce the tube structure formation and maintain the tube structure for up to 7 days.
Cell Invasion
![]()
Cell Invasion
Investigating cell mobility such as invasion and migration is important to study the metastatic cells. VitroGel is a powerful system for this study. Using the 2D hydrogel coating, scientists can monitor the cell behaviors when they pass through the hydrogel. The tunable and functional VitroGel system can be manipulated to study many different factors on cell mobility.
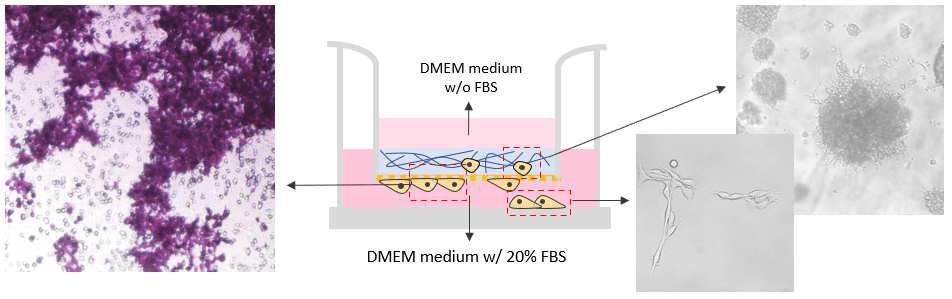
Invasion of B35 neuroblastoma cells on VitroGel system.
VitroGel RGD was used as 2D hydrogel coating on the surface as a cell insert. B35 cells were added directly on top of the hydrogel. The hydrogel was prepared at 1:3 dilution with 2% FBS. The cells were harvested from 2D culture plate with 10% FBS and resuspend in FBS free medium before adding to the top of the hydrogel. The medium outside of the insert well contained 20% FBS. The cell invasion was induced by the different concentrations of FBS in the cover medium, hydrogel, and the medium outside of the insert. The images were taken at day 7.
Read More on INVASION ASSAY