Injectable Hydrogel for In Vivo Applications
In Vivo Applications
Xeno-free VitroGel® Hydrogel
for Tissue Engineering
VitroGel xeno-free (animal-free) hydrogels are excellent for injection and a superior alternative to the animal-based extracellular matrix (ECM) for patient-derived or cell-derived xenograft (PDX & CDX) applications. By avoiding the uncertainty of unknown components from the animal-based ECM, VitroGel hydrogels give well-defined and full control of the microenvironment for consistent results.
Room temp stable
Ready-to-use. All protocols are at room temperature.
(10-20 prep time)

Xeno-free
100% animal origin-free system with batch-to-batch consistency.
Full ECM Control
Full control of the ECM supplements to boost cell growth. Neutral pH. Biodegradable and supports cell activities.
Long Injectable Status
Maintain an injectable status for hours at room temp or at 37°C.
How to select the functional hydrogel for your research project
Our xeno-free functional hydrogel system is designed based on the application needs (ready-to-use VitroGel) and the biochemical/biophysical properties needs (high concentration VitroGel). Scientists can choose the hydrogels that fit their research objectives.
All VitroGel hydrogels are injectable and excellent for tissue engineering. Researchers have full control of the supplement/growth factors in the hydrogel-cell mixture. Below are some hydrogel recommendations based on the popular applications:
> Ready-To-Use VitroGel
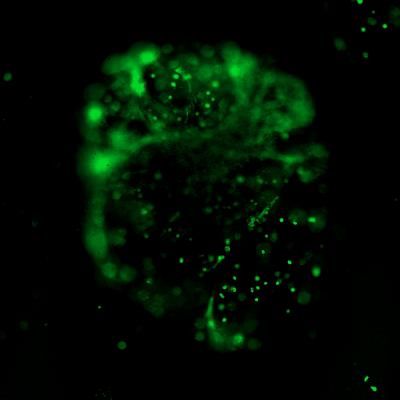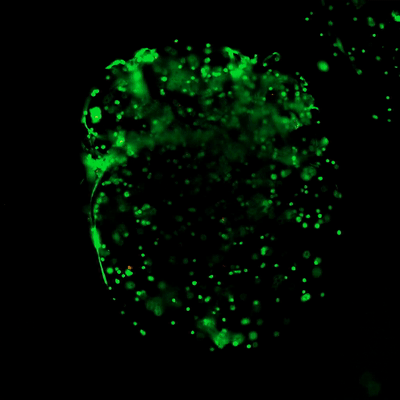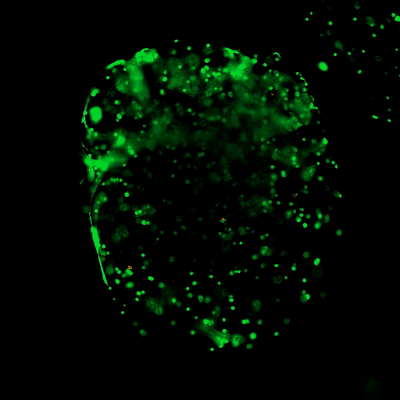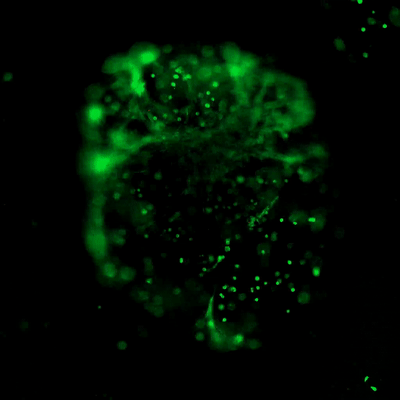
The ready-to-use VitroGel hydrogels have optimized formulations of multi-functional ligands and concentrations. Each hydrogel is ready to mix directly with cells/compound suspension, offering an excellent balance of simplicity and versatility. (Click the image on the right to view all different types of ready-to-use VitroGel)
- VitroGel® Hydrogel Matrix: Supports a wide variety of cell types including many cancer cells, epithelial cells, stromal cells, and primary cells. Great for cells needing strong cell-matrix interactions. (VHM01)
- VitroGel® ORGANOID-4: Supports tumor growth and is great for in vivo cell expansion. (VHM04-4)
- VitroGel® MSC: For Mesenchymal Stem Cells (MSCs) to attach and grow. A soft hydrogel for MSCs injection. (VHM03)
- VitroGel® STEM: For stem cell maintenance and expansion. Good for iPSC injection. (VHM02)
> High Concentration VitroGel
The high concentration hydrogels allow the flexibility to manipulate the mechanical strength of the hydrogel (tunable) by adjusting the hydrogel concentration with the VitroGel Dilution Solution. Each high-concentration hydrogel comes with a functional ligand modification allowing scientists to investigate the cell behaviors with complete control of hydrogel properties. (Click the image on the right to check all different types of high-concentration VitroGel)
- VitroGel® RGD: One of the most popular hydrogels for multiple in vivo applications. Supports a wide range of cell types with excellent cell-matrix interactions. Great for multiple cell therapy applications. (TWG003)
- VitroGel® MMP: Great hydrogel degradability to support rapid cell expansion, and invasion/migration. Excellent for tumor study and xenograft. (TWG010)
- VitroGel® 3D: Pure hydrogel matrix without binding ligand modification. Great for simple target delivery and control release. (TWG001)
Case Studies/Research Highlights
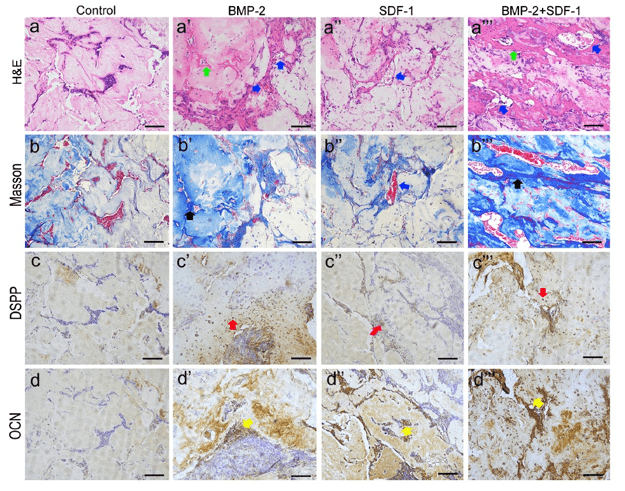
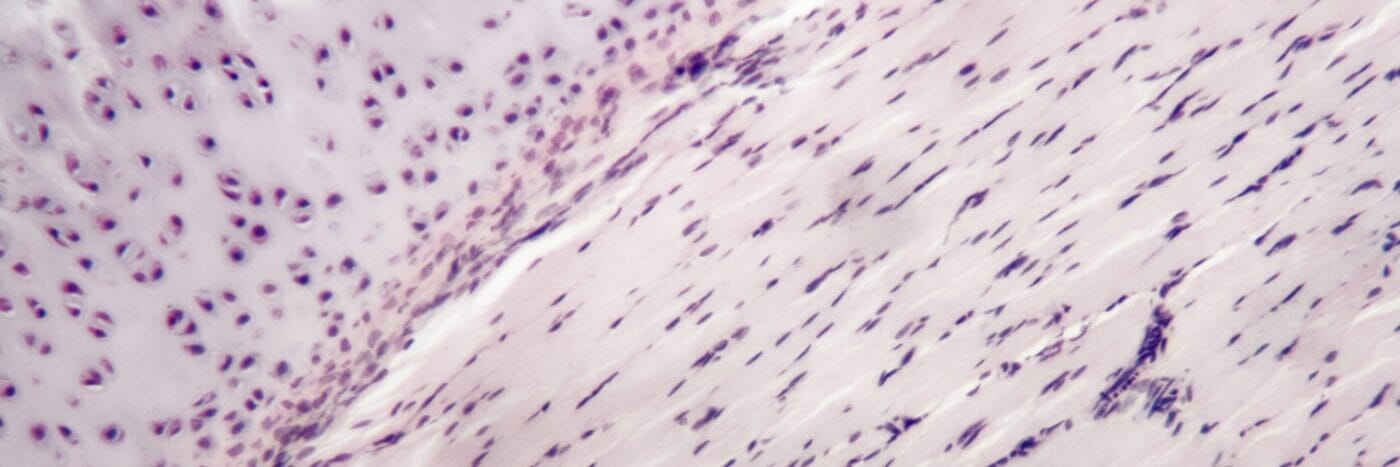
Getting to the root of the problem: Injectable VitroGel® 3D hydrogel scaffolds enhance odontogenic differentiation in vivo
The biocompatible and injectable VitroGel hydrogel culture system enhances pulp-dentin regeneration and has the potential for chair-side regenerative endodontic procedures
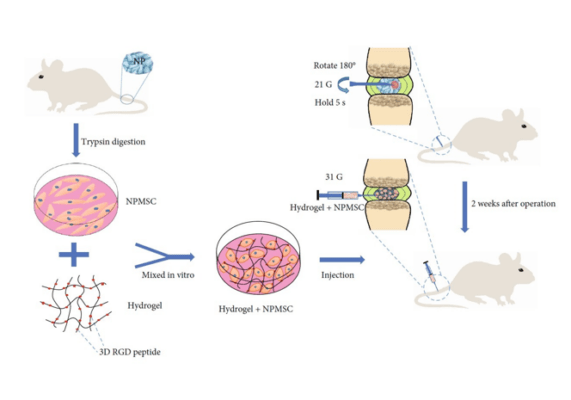
VitroGel’s ability to enhance the ECM-cell interaction enhances the transplantable capacity of MSCs in rat models of intervertebral disc regeneration
VitroGel® RGD enhances stem cell transplantation to treat intervertebral disc degeneration using rat models, laying new foundations in the movement toward cell-based tissue therapies.
READ MORE >
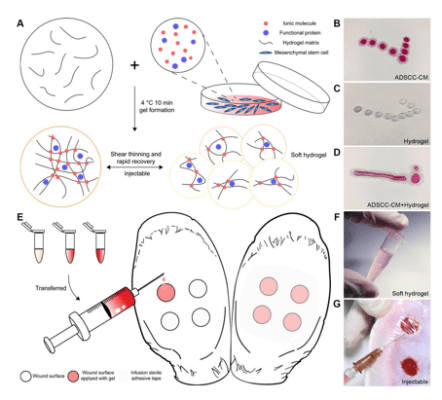
Inhibition of Scar Proliferation with In-situ Cross Bonding of VitroGel Hydrogel and Adipose-derived Stem Cells Conditioned Medium
Developing ADSCC-CM and VitroGel hydrogel complex to study hypertrophic scar prophylaxis.
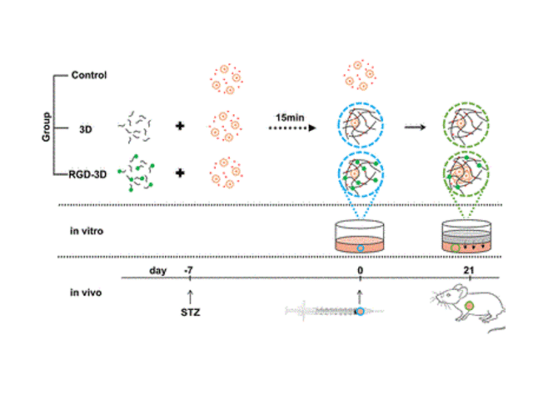 VitroGel Hydrogel Used to Bridge the Gap Between In Vivo and In Vitro Pancreatic Islet Beta-cells Studies
VitroGel Hydrogel Used to Bridge the Gap Between In Vivo and In Vitro Pancreatic Islet Beta-cells Studies
Scientists used VitroGel RGD to dramatically increase the survival rate and insulin secretory capacity of pancreatic islet beta-cells both in vitro and in vivo. The study provides a reference for using a xeno-free functional hydrogel system for islet transplantation.
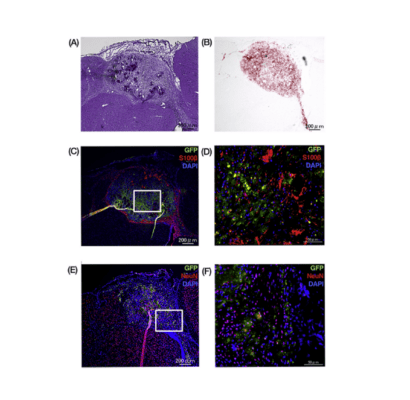
The injectable VitroGel provides a viable in vivo scaffold for novel maternal-fetal adipose stem cell transplant studies
Maternally derived adipose stem cells have emerged as a possible solution to the common immunological rejection observed in fetal repair surgeries.
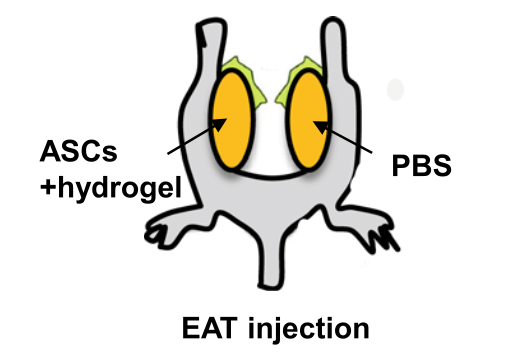
Glucose Metabolism in Obese Mice can be Improved by Implantation with Photoactivated Adipose Stem Cells
VitroGel was used as an injectable adjuvant for in vivo implantation of adipose stem cells to ameliorate glucose intolerance in obese mice.
Research publications Using VitroGel for in vivo applications
- Ricotti, L., Cafarelli, A., Manferdini, C., Trucco, D., Vannozzi, L., Gabusi, E., Fontana, F., Paolo Dolzani, Saleh, Y., Lenzi, E., Columbaro, M., Piazzi, M., Bertacchini, J., Aliperta, A., Cain, M. G., Gemmi, M., Parlanti, P., Jost, C., Yirij Fedutik, & Gilbert Daniel Nessim. (2024). Ultrasound Stimulation of Piezoelectric Nanocomposite Hydrogels Boosts Chondrogenic Differentiation in Vitro, in Both a Normal and Inflammatory Milieu. ACS Nano. https://doi.org/10.1021/acsnano.3c08738
- Mavinga, M., Palmier, M., Rémy, M., Jeannière, C., Lenoir, S., Rey, S., Saint-Marc, M., Alonso, F., Génot, E., Thébaud, N., Chevret, E., Mournetas, V., Rousseau, B., Boiziau, C., & Boeuf, H. (2022). The Journey of SCAPs (Stem Cells from Apical Papilla), from Their Native Tissue to Grafting: Impact of Oxygen Concentration. Cells, 11(24), 4098. https://doi.org/10.3390/cells11244098
- Manferdini, C., Trucco, D., Saleh, Y., Gabusi, E., Dolzani, P., Lenzi, E., Vannozzi, L., Ricotti, L., & Lisignoli, G. (2022). RGD-Functionalized Hydrogel Supports the Chondrogenic Commitment of Adipose Mesenchymal Stromal Cells. Gels, 8(6), 382. https://www.mdpi.com/2310-2861/8/6/382/
- Mori, H., Saeki, K., Chang, G., Wang, J., Wu, X., Hsu, P.-Y., Kanaya, N., Wang, X., Somlo, G., Nakamura, M., Bild, A., & Chen, S. (2021). Influence of Estrogen Treatment on ESR1+ and ESR1− Cells in ER+ Breast Cancer: Insights from Single-Cell Analysis of Patient-Derived Xenograft Models. Cancers, 13(24), 6375. https://doi.org/10.3390/cancers13246375
- Zhang, C., Wang, T., Zhang, L., Chen, P., Tang, S., Chen, A., Li, M., Peng, G., Gao, H., Weng, H., Zhang, H., Li, S., Chen, J., Chen, L., & Chen, X. (2021). Combination of lyophilized adipose-derived stem cell concentrated conditioned medium and polysaccharide hydrogel in the inhibition of hypertrophic scarring. Stem Cell Research & Therapy, 12(1). https://doi.org/10.1186/s13287-020-02061-3
- Lan, T., Guo, J., Bai, X., Huang, Z., Wei, Z., Du, G., Yan, G., Weng, L., & Yi, X. (2020). RGD-modified injectable hydrogel maintains islet beta-cell survival and function. Journal of Applied Biomaterials & Functional Materials,18, 228080002096347. https://doi.org/10.1177/2280800020963473
- Zhu, L., Feng, Z., Shu, X., Gao, Q., Wu, J., Du, Z., Li, R., Wang, L., Chen, N., Li, Y., Luo, M., & Wu, J. (2021). In situ transplantation of adipose-derived stem cells via photoactivation improves glucose metabolism in obese mice. Stem Cell Research & Therapy, 12,1. https://doi.org/10.1186/s13287-021-02494-4
- Jiang, Y., Li, S., Zhou, Q., Liu, S., Liu, X., Xiao, J., Jiang, W., Xu, Y., Kong, D., Wang, F., Wei, F., & Zheng, C. (2021). PDCD4 Negatively Regulated Osteogenic Differentiation and Bone Defect Repair of Mesenchymal Stem Cells Through GSK-3β/β-Catenin Pathway. Stem Cells and Development. https://doi.org/10.1089/scd.2021.0041
- Tian, X., Song, J., Zhang, X., Yan, M., Wang, S., Wang, Y., Xu, L., Zhao, L., Wei, J., Shao, C., Kong, B., & Liu, Z. (2020). MYC-regulated pseudogene HMGA1P6 promotes ovarian cancer malignancy via augmenting the oncogenic HMGA1/2.Cell Death & Disease, 11(3), 1–14. https://doi.org/10.1038/s41419-020-2356-9
- Kawashima, A., Yasuhara, R., Akino, R., Mishima, K., Nasu, M., & Sekizawa, A. (2020). Engraftment potential of maternal adipose-derived stem cells for fetal transplantation.Heliyon, 6(3), e03409. https://doi.org/10.1016/j.heliyon.2020.e03409
- Xiao, M., Qiu, J., Kuang, R., Zhang, B., Wang, W., & Yu, Q. (2019). Synergistic effects of stromal cell-derived factor-1 α and bone morphogenetic protein-2 treatment on odontogenic differentiation of human stem cells from apical papilla cultured in the VitroGel 3D system. Cell and Tissue Research, 378(2), 207–220. https://doi.org/10.1007/s00441-019-03045-3
- Wang, F., Nan, L., Zhou, S., Liu, Y., Wang, Z., Wang, J., Feng, X., & Zhang, L. (2019). Injectable Hydrogel Combined with Nucleus Pulposus-Derived Mesenchymal Stem Cells for the Treatment of Degenerative Intervertebral Disc in Rats. Stem Cells International, 2019, 1–17. https://doi.org/10.1155/2019/8496025