Quick and Efficient Organoid/Cell Recovery from Animal-Based Extracellular Matrix
VitroGel® Cell Recovery Solution (100 mL)
Enzyme-free cell harvesting solution to recover cells from the hydrogel in 20 min.
View the improved version that can also recover cells from animal-based ECM.
$72.90
VitroGel Cell Recovery Solution – Cell Harvesting
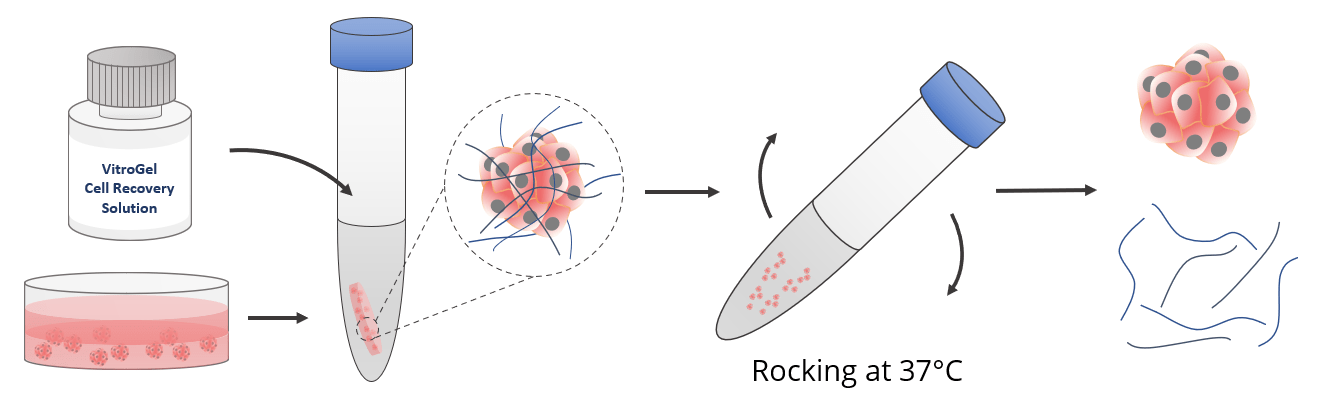
VitroGel® Cell Recovery Solution is an non-enzymatic cell harvesting solution to recover cells/organoids cultured in 2D, 3D efficiently and safely in 20 minutes.
VitroGel Cell Recovery Solution is room temperature stable, has a neutral pH, and works at 37°C operating temperature. The solution can maintain high cell viability during the recovery process. Harvested cells can be sub-culture in both 2D and 3D cultures.
The VitroGel Cell Recovery Solution can be used before or after the fixation and stained preparation of hydrogel specimens to ensure high-quality downstream data analysis.
Easy cell recovery from hydrogel in 20 minutes.
Specifications
| Size | 100 mL |
| Formulation | Enzyme-free |
| Use | Harvest cells from VitroGel hydrogel while maintaining high cell viability. Use before or after sample fixation and stained preparation for imaging or downstream data analysis |
| Processing Time | 15-20 min |
| Downstream | Recovered cells can be sub-culture in both 2D and 3D culture |
| pH | Neutral |
| Storage | Ambient Temperature (15-30°C) |
| Stability | 60 months from date of manufacture |
Protocols / Resources
Video Protocols & Demonstrations
TECHNICAL TIPS
- KEEP SOLUTION WARM: It is important to keep the cell recovery solution and the mixture warm at 37°C during the whole process. The warm temperature is essential to accelerate molecular exchanges to release the ionic molecules from the solid hydrogel, which can transform into a soft hydrogel.
- APPLY MECHANICAL FORCE: The mechanical force, such as rocking or shaking the centrifuge tube or using a serological pipette to mix the hydrogel with the cell recovery solution helps to transform the hydrogel into the liquid state.
- DILUTION: Adding the cell recovery solution at a volume of 10X or higher than the hydrogel maintains the dissolved hydrogel in a liquid state.
- CENTRIFUGE AT ROOM TEMPERATURE
Application Notes
APPLICATION NOTE
Data and References
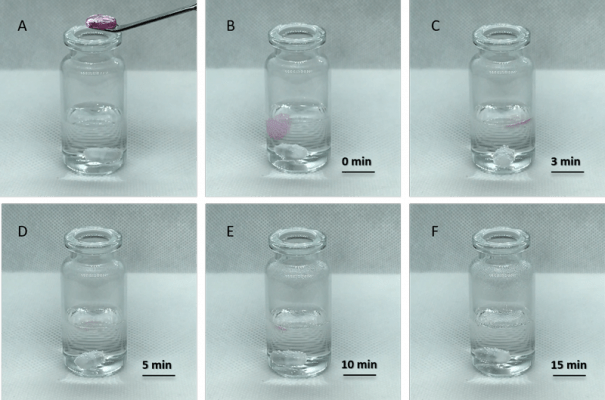
Figure 1. Fast hydrogel dissolved in VitroGel Cell Recovery Solution.
A. Hydrogel before adding to recovery solution; B-F. Time 0 to 15 min after adding hydrogel to recovery solution (at 37 °C, 20 rpm).
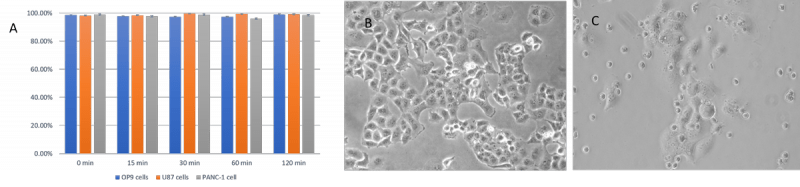
Figure 2 The VitroGel Cell Recovery Solution maintains high cell viability
A. Cell viability of OP9, U87-MG and PANC-1 cells after adding to recovery solution at time 0, 15, 30, 60 and 120 min. Cells maintain over 95% cell viability after suspending in VitroGel cell recovery solution for 2 hr.; B. PANC-1 cells growth on 2D well plate before transfer to cell recovery solution; C. PANC-1 cells suspended in cell recovery solution for 24 hours then re-culture on 2D well plate for 5 days. Cells has been successful re-culture after suspend in cell recovery solution for 24 hours.
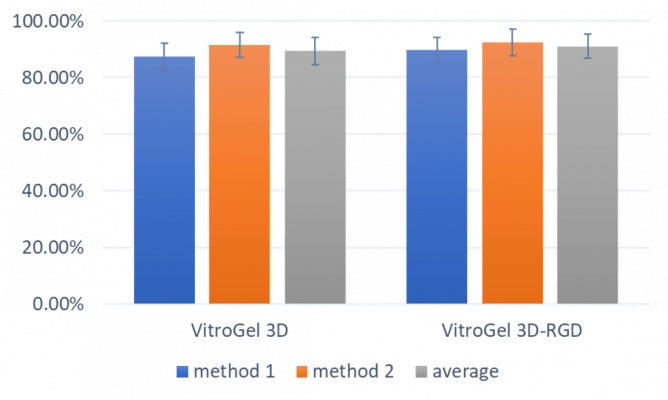
Figure 3.
Cell viability of 3D cultured PANC-1 cells after recovering from hydrogel. (Method 1: add whole gel into cell recovery solution. Method 2: using pipette to break gel into small piece before adding into cell recovery solution. Average: the average cell viability of method 1 and method 2.)
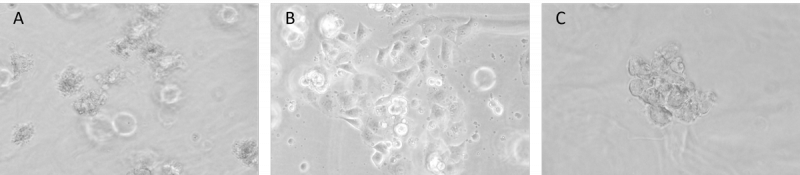
Figure 4. Cells can be sub-culture in both 2D and 3D culture after recovery. A.
PANC-1 cells growth on 3D hydrogel before harvested by VitroGel cell recovery solution; B. PANC-1 cells have been harvested from 3D hydrogel by using VitroGel cell recovery solution and subculture on the surface of hydrogel (day 2); C. PANC-1 cells have been harvested from 3D hydrogel by using the cell recovery solution and 3D subculture in the hydrogel system again (day 2).
References/Publications
- Gabusi, E., Lenzi, E., Manferdini, C., Dolzani, P., Columbaro, M., Saleh, Y., & Lisignoli, G. (2022). Autophagy Is a Crucial Path in Chondrogenesis of Adipose-Derived Mesenchymal Stromal Cells Laden in Hydrogel. Gels , 8(12),766. https://www.mdpi.com/2310-2861/8/12/766
- De Donato, M., Babini, G., Mozzetti, S., Buttarelli, M., Ciucci, A., Arduini, G., De Rosa, M. C., Scambia, G., & Gallo, D. (2020). KLF7: a new candidate biomarker and therapeutic target for high-grade serous ovarian cancer. Journal of Experimental & Clinical Cancer Research, 39(1). https://doi.org/10.1186/s13046-020-01775-9
- Lan, T., Guo, J., Bai, X., Huang, Z., Wei, Z., Du, G., Yan, G., Weng, L., & Yi, X. (2020). RGD-modified injectable hydrogel maintains islet beta-cell survival and function. Journal of Applied Biomaterials & Functional Materials, 18, 228080002096347. https://doi.org/10.1177/2280800020963473
- Powell K. Adding depth to cell culture. Science, 356(6333), 96–98. https://doi.org/10.1126/science.356.6333.96
- References to all VitroGel hydrogels >
| Size | 100 mL |
|---|