Xeno-free and biofunctional
100% synthetic. Animal & human origin-free hydrogel. No growth factors or proteins in the gel solution.
VitroGel® Hydrogel Matrix
ready-to-use, xeno-free (animal origin-free) biofunctional hydrogel system
VitroGel® Hydrogel Matrix – 3D Cell Culture


Stable at room temperature. 20-min protocol.
Simple, fast, and easy-to-use. Room temperature protocol/operation. No ice bucket.

Supports a wide range of cell types and applications.
Ideal for 2D/3D, organoids, stem cells, MSCs, spheroids, functional assay, co-culture, and PDX and CDX in vivo application, and more.
Easy cell harvesting
Easy, efficient, and enzyme-free cell harvesting in 20 min.
VitroGel® Hydrogel Matrix is a ready-to-use, xeno-free (animal origin-free) functional hydrogel for 3D cell culture research. VitroGel® Hydrogel Matrix is an optimized formulation of multi-functional ligands and concentrations to support a wide range of cell types for different applications.“Just Add Cells” – The hydrogel matrix is ready to mix with cell suspension directly.
There is no additional adjustment needed. VitroGel® Hydrogel Matrix closely mimics the natural extracellular matrix (ECM) environment to make cells feel more like at home. The hydrogel is room temperature stable, has a neutral pH, transparent, permeable and compatible with different imaging systems. The solution transforms into a hydrogel matrix by simply mixing with the cell culture medium. Cells cultured in this system can be easily harvested out with our VitroGel® Organoid Recovery Solution. This user-friendly functional hydrogel creates an excellent balance of simplicity and versatility.
Specifications
| Formulation | Xeno-free, functional hydrogel |
| Use | 3D and 2D cell culture |
| Operation | Ready-to-use at room temperature |
| Biocompatibility | Biocompatible, safe for animal studies |
| Injection | Injectable hydrogel for in vivo studies and lab automation |
| pH | Neutral |
| Storage | Store at 2-8°C. Ships at ambient temperature |
| Sizes | 10 mL and 2 mL |
| Number of Uses | (10 mL) 300 uses at 50 µL per well (2 mL) 60 uses at 50 µL per well |
| Complementary Products | Cell Harvesting VitroGel Organoid Recovery Solution 10-15 min cell recovery Cell Viability Cyto3D Live-Dead Assay Kit Fast, sensitive |
3D cell culture process in 20 min – “Just add cells”
VitroGel® Hydrogel Matrix is ready to use. Just mix with your cells. There is no cross-linking agent or the need to adjust the hydrogel concentration.
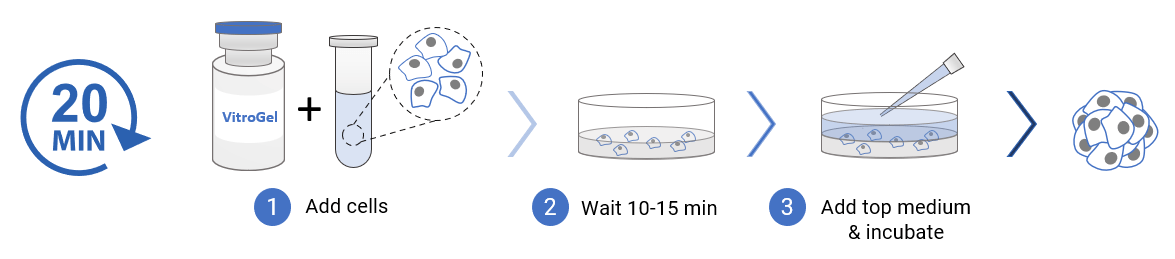
Protocols and Resources
Webinars
Application Notes
Research Highlights and White Papers
Data and References
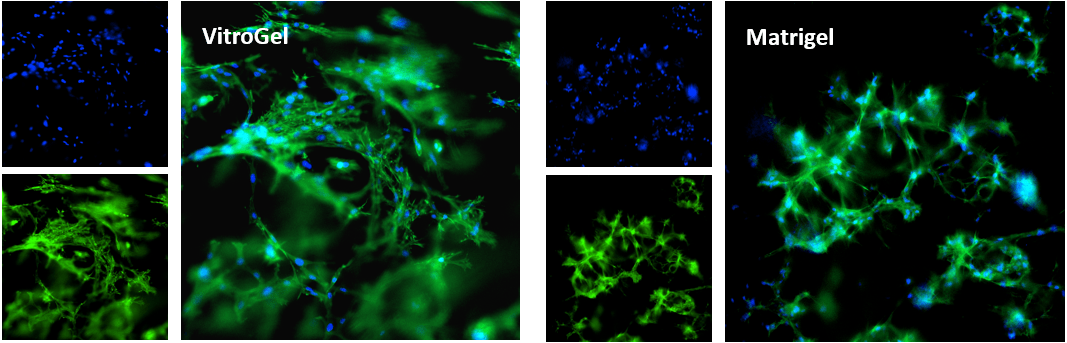
Figure 1. Bone marrow cells 3D cultured in VitroGel® Hydrogel Matrix and Matrigel, a natural Extracellular Matrix (ECM) hydrogel
The fibroblast-like mouse bone marrow stromal cells (OP9-GFP) were 3D cultured in VitroGel® Hydrogel Matrix and Matrigel®, a natural ECM-based hydrogel from Engelbreth-Holm-Swarm murine sarcoma. Single cells were homogenously suspended within each hydrogel, with each forming stretched fibroblast-like structure on day 1. The images above shows a clear 3D cellular networking structure formed in both hydrogels on day 7. Compared to Matrigel, the multiple functional ligands in the VitroGel® Hydrogel Matrix promote a stronger cell-matrix interaction, which helps accelerate the cell proliferation and cell-cell communication during the 3D cell culture.
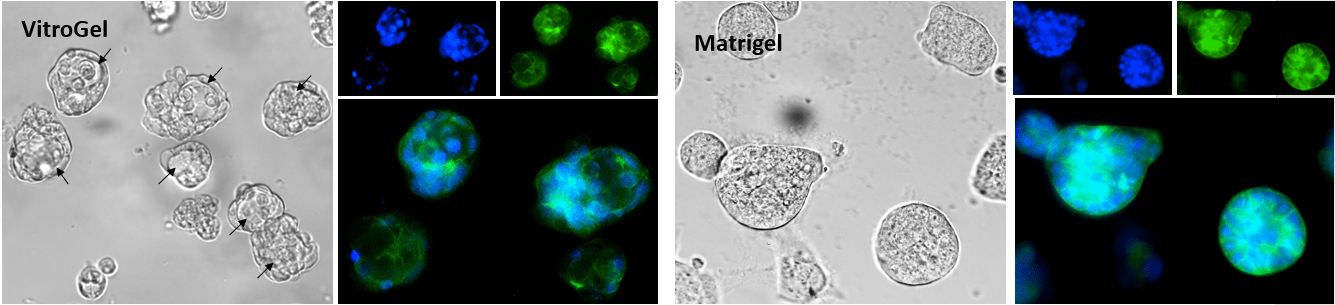
Figure 2. Human mammary breast cancer cells cultured in VitroGel® Hydrogel Matrix and Matrigel, a natural Extracellular Matrix (ECM) hydrogel
VitroGel® Hydrogel Matrix can support the growth of various cell types. The images above are 3D cell culture of human mammary breast cancer cells (MCF-7) on day 7 in VitroGel® Hydrogel Matrix and Matrigel®, a natural ECM-based hydrogel from Engelbreth-Holm-Swarm murine sarcoma. The cells were prepared as single-cell suspensions and encapsulated within each hydrogel respectively. The grape-shaped like cell colonies appeared on day 1 for both hydrogels. However, cells displayed 3D luminal structures (see arrows) only within the VitroGel® Hydrogel Matrix. The cells cultured in Matrigel can only perform the spheroid structure. (Z-stack imaging system with 2D image projection was used. Blue: DAPI; Green: ActinGreen™)
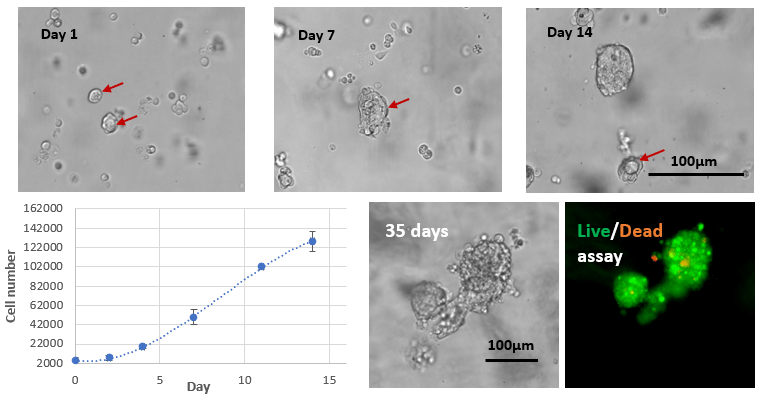
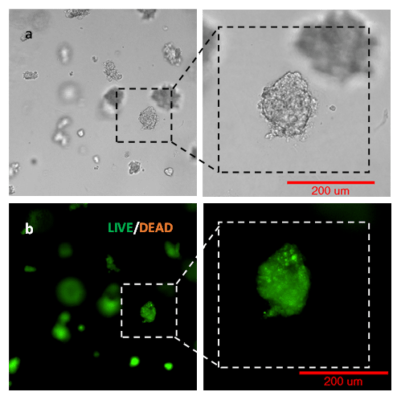
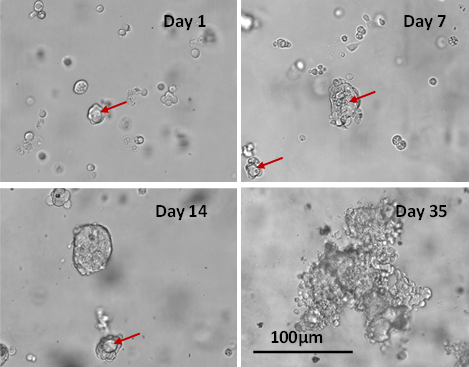
Fig 3. Long term 3D cell culture of MCF7 breast cancer cells in VitroGel® Hydrogel Matrix
The images above show the cells at different stages of the long-term culture on days 1, 7, 14, and 35. The formation of the lumen structures were observed by day 7. By day 14, cellular polarity loss gave rise to spheroid structures. The malignant stage of the spheroid continued to produce the heterogenous mass of cells observed on day 35. The live/dead assay shows disorganized metastatic organization of cells within the tumor while the viability image shows a small zone of dead cell (red) at the center of the spheroid as well as surrounding the sphere.
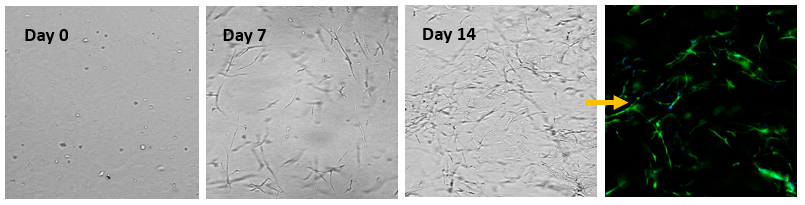
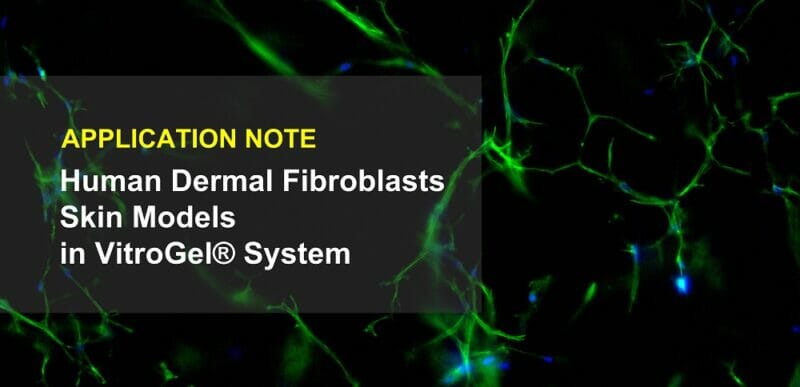
Fig 4. 3D cell culture of normal Human Dermal Fibroblast (NHDF) cells in VitroGel® Hydrogel Matrix
Human Dermal Fibroblast (NHDF) cells were encapsulated within the VitroGel® Hydrogel Matrix and cultured for 14 days. The images above show the 3D networking of the fibroblast structures, indicating strong matrix-cell interactions.
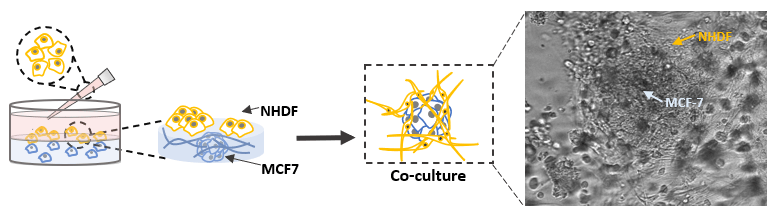
Fig 5. Co-culture of human mammary breast cancer cells (MCF-7) and normal Human Dermal Fibroblast (NHDF) cells in VitroGel® Hydrogel Matrix
MCF-7 cells were encapsulated in a hydrogel matrix by mixing the cell suspension with VitroGel® Hydrogel Matrix. After allowing the mixture to stabilize at room temperature for 10 min, NHDF cells were added on top of the hydrogel for 2D hydrogel coating culture. The NHDF cells attached and moved inside the hydrogel matrix after 48 hours of culture. The NHDF cells grew surrounding the MCF-7 spheroids and supported the fast growth of MCF-7 for large tumor structure formation.
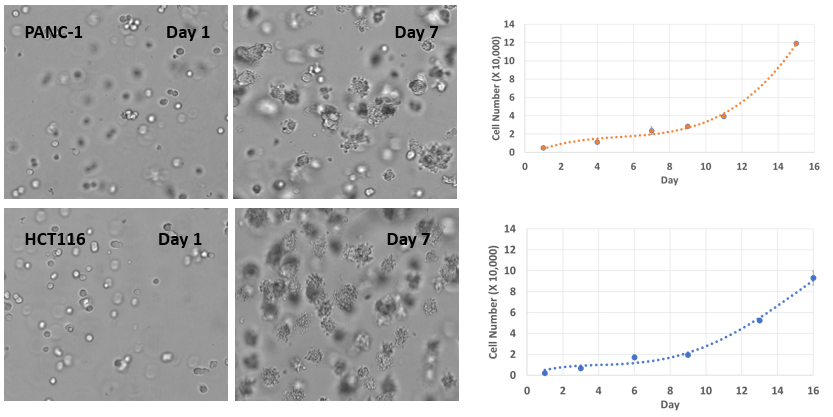
Fig 6. 3D cell culture of pancreatic cancer cells (PANC-1) and colon cancer cells (HCT116) in VitroGel® Hydrogel Matrix
PANC-1 and HCT116 cells were seeded within VitroGel® Hydrogel Matrix as single cells. The images above show both cell type’s rapid growth and tumor spheroid formations in the VitroGel® Hydrogel Matrix. The hydrogel system is suitable for long-term cell culture for more than 21 days.
RELATED APPLICATION NOTE FOR FIGURE 6. READ MORE >
Matrigel is a trademark of Corning Incorporated.
References/Publications
- Benman, W., Huang, Z., Iyengar, P., Wilde, D., Mumford, T. R., & Bugaj, L. J. (2025). A temperature-inducible protein module for control of mammalian cell fate. Nature Methods. https://doi.org/10.1038/s41592-024-02572-4
- Lotfollahzadeh, S., Yang, X., Wu Wong, D. J., Han, J., Seta, F., Ganguli, S., Jose, A., Ravid, K., & Chitalia, V. C. (2024). Venous Thrombosis Assay in a Mouse Model of Cancer. Journal of Visualized Experiments, 203. https://doi.org/10.3791/65518
- Venuta, A., Nasso, R., Gisonna, A., Iuliano, R., Montesarchio, S., Acampora, V., Sepe, L., Avagliano, A., Arcone, R., Arcucci, A., & Ruocco, M. R. (2023). Celecoxib, a Non-Steroidal Anti-Inflammatory Drug, Exerts a Toxic Effect on Human Melanoma Cells Grown as 2D and 3D Cell Cultures. Life, 13(4), 1067. https://doi.org/10.3390/life13041067
- Tsai, Y.-C., Kung Hung Cheng, Shih Sheng Jiang, Hawse, J. R., Shun En Chuang, Su Liang Chen, Huang, T.-S., & Hui-Ju Ch’ang. (2023). Krüppel-like factor 10 modulates stem cell phenotypes of pancreatic adenocarcinoma by transcriptionally regulating notch receptors. Journal of Biomedical Science, 30(1). https://doi.org/10.1186/s12929-023-00937-z
- Mavinga, M., Palmier, M., Rémy, M., Jeannière, C., Lenoir, S., Rey, S., Saint-Marc, M., Alonso, F., Génot, E., Thébaud, N., Chevret, E., Mournetas, V., Rousseau, B., Boiziau, C., & Boeuf, H. (2022). The Journey of SCAPs (Stem Cells from Apical Papilla), from Their Native Tissue to Grafting: Impact of Oxygen Concentration. Cells, 11(24), 4098. https://doi.org/10.3390/cells11244098
- Niwa, R., Hanamatsu, Y., Kito, Y., Saigo, C., & Takeuchi, T. (2022). Experimental model of micronodular thymic neoplasm with lymphoid stroma. Thoracic Cancer. https://doi.org/10.1111/1759-7714.14716
- Olofsen, P. A., Stip, M. C., Jansen, J. H. M., Chan, C., Nederend, M., Tieland, R. G., Tsioumpekou, M., & Leusen, J. H. W. (2022). Effective, Long-Term, Neutrophil Depletion Using a Murinized Anti-Ly-6G 1A8 Antibody. Cells, 11(21), 3406. https://doi.org/10.3390/cells11213406
- Bhatt R., et al. Scaffold-mediated switching of lymphoma metabolism in culture. Cancer & Metabolism. https://doi.org/10.1186/s40170-022-00291-y
- Ishikawa-Ankerhold, H., et al. (2022). Centrosome Positioning in Migrating Dictyostelium Cells. Cells. https://www.mdpi.com/2073-4409/11/11/1776/htm
- Powell K. Adding depth to cell culture. Science, 356(6333), 96–98. https://doi.org/10.1126/science.356.6333.96
| Size | 2 mL, 10 mL |
|---|