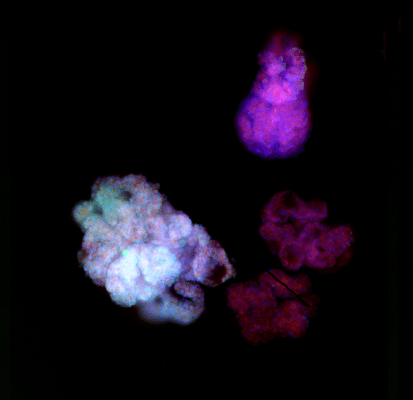Xeno-free hydrogel for organoid formation
100% synthetic. Animal & human origin-free, biofunctional hydrogel.
VitroGel® ORGANOID
ready-to-use, xeno-free (animal origin-free) hydrogel system for organoid culture
VitroGel® ORGANOID
Ready-to-use, xeno-free hydrogel for organoid formation and expansion.

Supports a wide range of organoids
Ideal for organoids from patient-derived samples, stem cells, tissues, co-culture and PDX resources.

Stable at room temperature. 20-min protocol.
Simple, fast, and easy-to-use. Room temperature protocol/operation. No ice bucket.
Easy cell harvesting
Simple and efficient cell harvesting by either centrifuge or the non-enzymatic VitroGel® Organoid Recovery Solution.
VitroGel® ORGANOID (1-4) are xeno-free (animal origin-free) hydrogels that support the growth of patient-derived organoids or organoids developed from pluripotent stem cells (PSCs), co-culture, and PDX model.
VitroGel® ORGANOID hydrogels are ready to use at room temperature and have a neutral pH, transparent, permeable, and compatible with different imaging systems. The solution transforms into a hydrogel matrix by simply mixing with the cell culture medium. VitroGel® ORGANOID hydrogels are good for both 3D cell culture and 2D hydrogel coating applications.
The VitroGel® ORGANOID hydrogels can work in conjunction with VitroGel® STEM (a xeno-free hydrogel for 3D static suspension cultures and scale-up of human pluripotent stem cells) by transferring stem cell spheroids cultured in VitroGel® STEM to VitroGel® ORGANOID hydrogels for organoid differentiation. See Xeno-free 3D Organoid Workflow . Key growth factors and molecules can be directly mixed with the hydrogel matrix or by adding on the top of the hydrogel. Organoids cultured in this system can be easily harvested out with our VitroGel® Organoid Recovery Solution. VitroGel® ORGANOID hydrogels provide a well-defined 3D microenvironment for the future of personalized medicine.
Specifications
| Formulation | Xeno-free, functional hydrogel |
| Use | Organoid culture |
| Operation | Ready-to-use at room temperature |
| Biocompatibility | Biocompatible, safe for animal studies |
| Injection | Injectable hydrogel for in vivo studies and lab automation |
| Cell Harvesting | VitroGel Organoid Recovery Solution 5-15 min cell recovery |
| pH | Neutral |
| Storage | Store at 2-8°C. Ships at ambient temperature |
| Sizes | Single Vial: 10 mL Discovery Kit: (2 mL) each of ORGANOID-1, ORGANOID-2, ORGANOID-3, ORGANOID-4 |
| Number of Uses | (10 mL): 600 uses at 25 µL per dome or 300 uses at 50 µL per well (2 mL): 120 uses at 25 µL per dome or 60 uses at 50 µL per well |
VitroGel® ORGANOID-1, VitroGel® ORGANOID-2, VitroGel® ORGANOID-3, VitroGel® ORGANOID-4)
 3D organoid cell culture process in 20 min
3D organoid cell culture process in 20 min
“Just add cells”
VitroGel® ORGANOID is ready-to-use. Just mix with your cells. There is no cross-linking agent or the need to adjust the hydrogel concentration.
Video Protocols
Application Notes and Research Highlights
VitroGel® Organoid Culture Methods
VitroGel® ORGANOID system can culture organoids in variety of methods:
3D DOME | 2D DOME | 2D Hydrogel Coating | 3D Cell Encapsulation | 3D Hydrogel-Cell Droplet
Review all the following VitroGel® ORGANOID protocol that best fits your project.
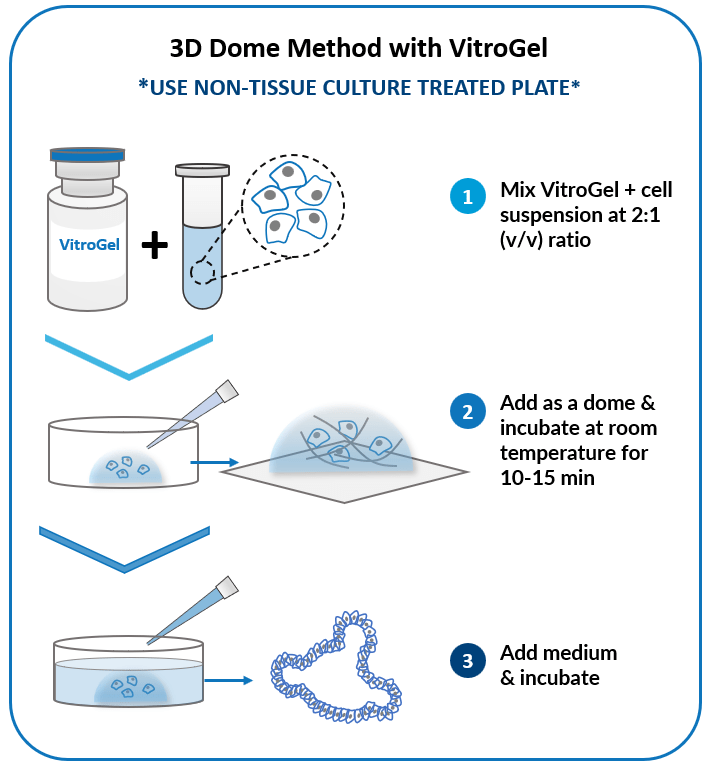
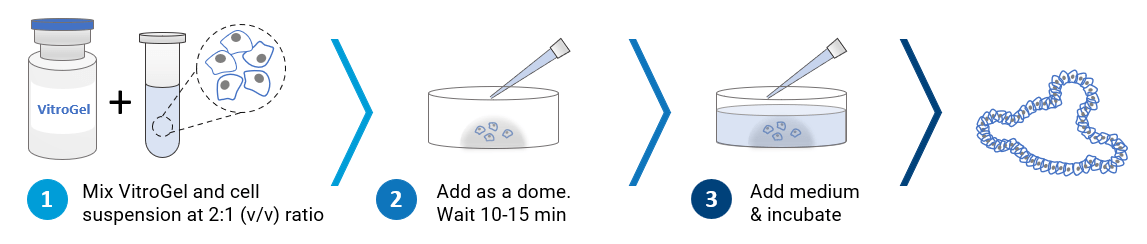
3D Dome Method
Widely used method for organoid generation.
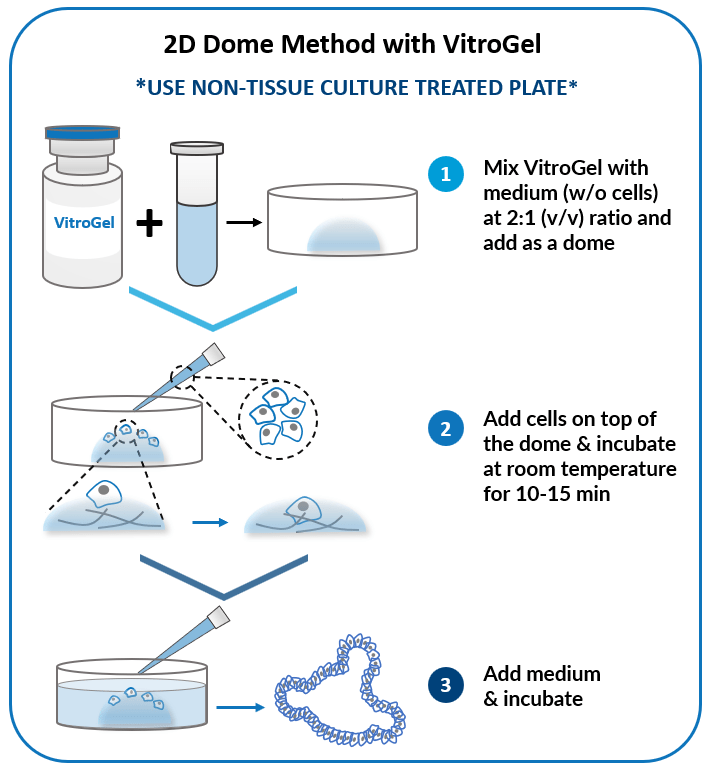
2D Dome Method
Add cells on top of the hydrogel dome for
organoid-gel-medium interactions.
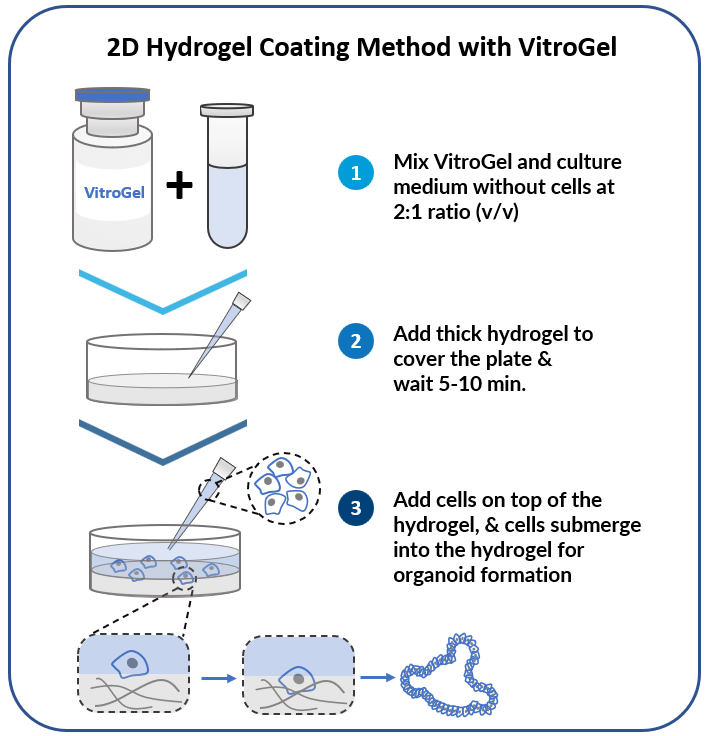
Add cells on top of hydrogel for optimal interactions among cells, hydrogel, and medium.
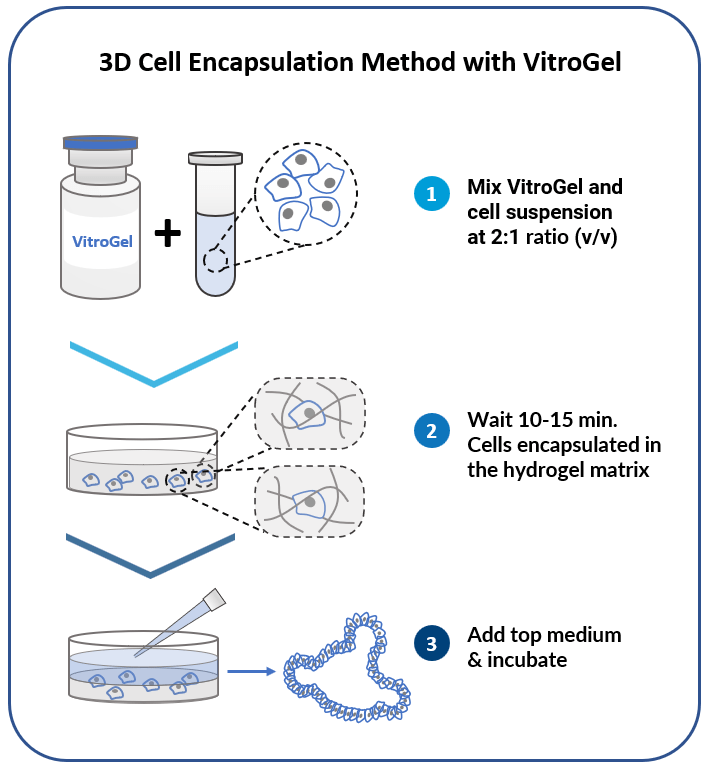
3D Cell Encapsulation Method
Encapsulate cells within the hydrogel for excellent mechanical support and the cell-matrix interactions.
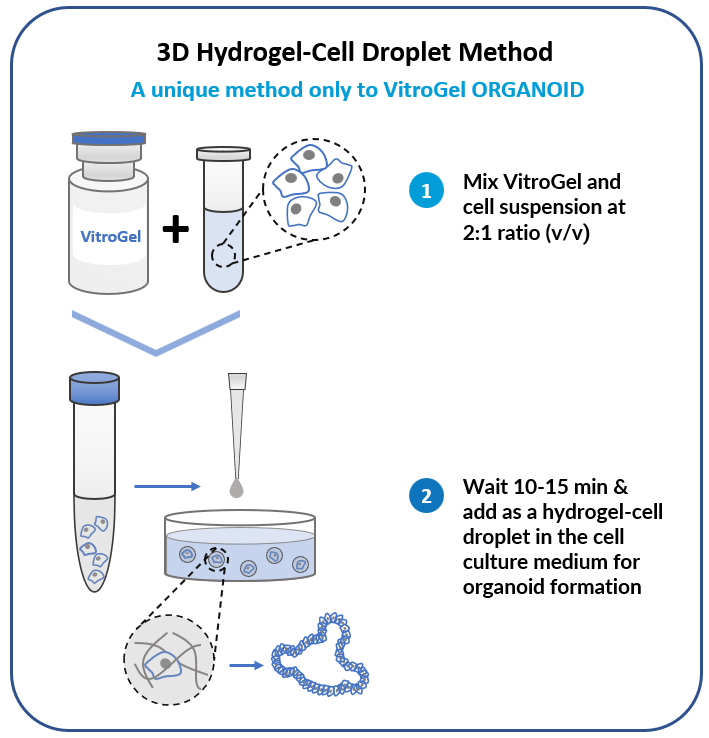
3D Hydrogel-Cell Droplet Method
Create a Hydrogel-Cell droplet. This is a unique culture method only to the VitroGel system for generating great cell-matrix interactions and excellent medium penetration for organoid formation and scale-up.
Data and References
Xeno-free 3D Organoid Workflow A:
Using VitroGel® ORGANOID for organoid culture and maturation
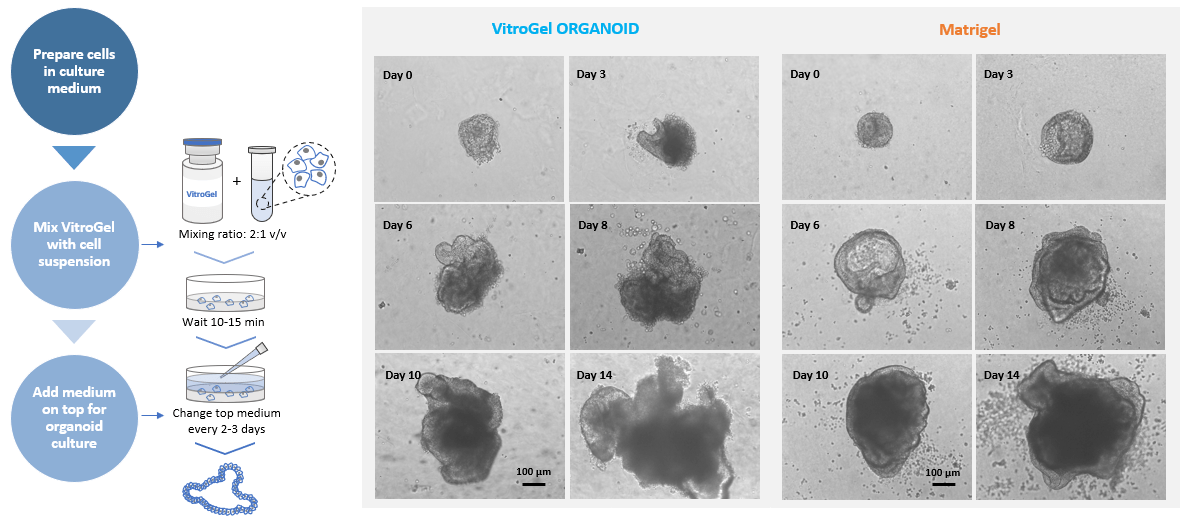
Figure 1. MOUSE INTESTINAL ORGANOID culture on VitroGel® ORGANOID and Matrigel.
Small organoids recovered from liquid nitrogen were directly seeded with VitroGel® and Matrigel, respectively.
2D Hydrogel Coating Method was used for VitroGel®. Images show the growth of mouse intestinal organoid from day 0 to day 14.
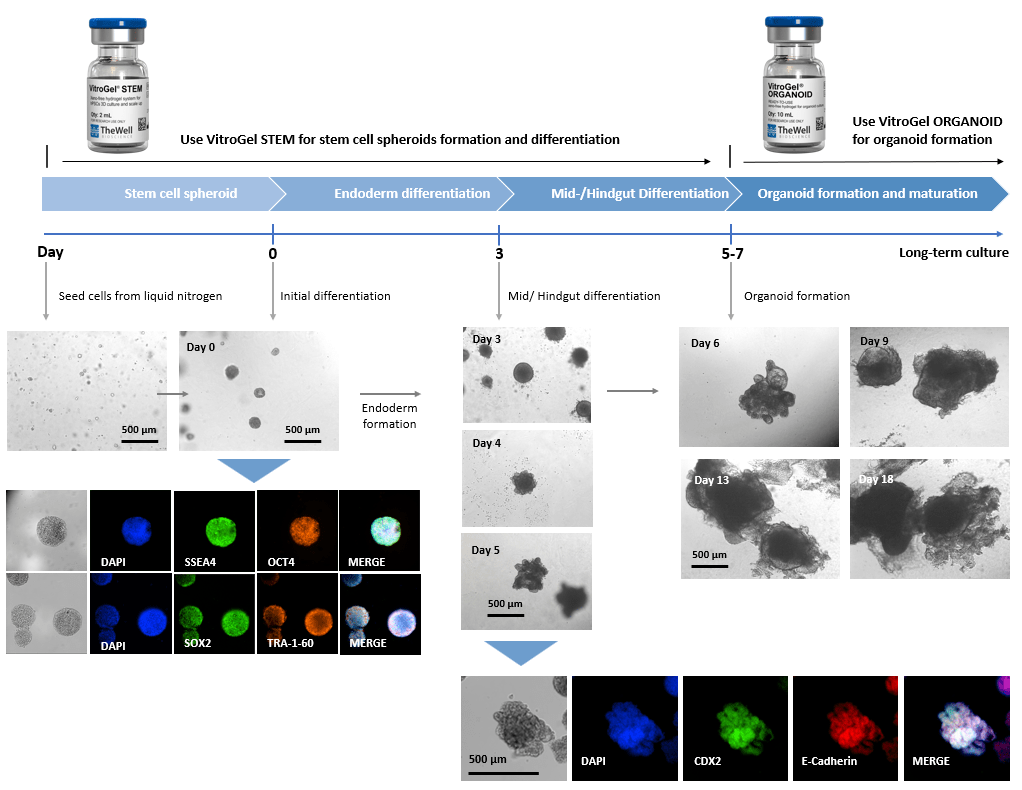
Xeno-free 3D Organoid Workflow B:
Start from iPSC spheroids for stem cell differentiation and organoid formation.
(Diagram below shows culturing human intestinal organoid from stem cell spheroids)
Figure 2. Culture human intestinal organoid from stem cell spheroids.
Human iPSCs recovered from liquid nitrogen were seeded with VitroGel® STEM for static suspension culture (Refer to protocol of VitroGel® STEM Cat# VHM02). The high-quality stem cell spheroids formed within 3-5 days with full pluripotent properties (showing the positive markers of SSEA4, OCT4, SOX2, and TRA-1-60). The spheroids were harvested by centrifuging (100 x g, 3 min), and resuspended with VitroGel® STEM in endoderm differentiation medium for 3 days. The endoderm cell spheroids were then harvested by centrifuging (100 x g, 3 min), and resuspended with VitroGel® STEM in mid/hindgut differentiation medium for 3-4 days. The mid/hindguts were collected by centrifuging (100 x g, 3 min), and characterized with CDX2 and E-Cadherin. The mid/hindguts were resuspended with organoid formation medium and mixed with VitroGel® ORGANOID (following the protocol of VitroGel® ORGANOID) for organoid formation and long-term maturating culture.
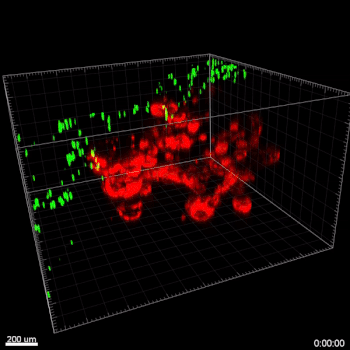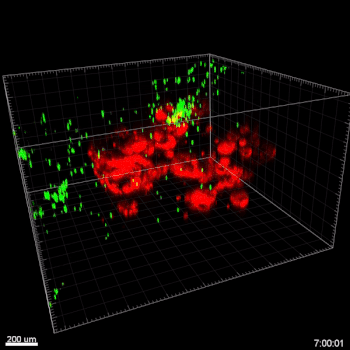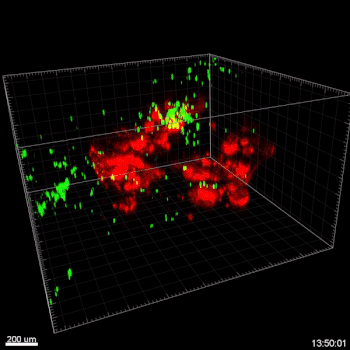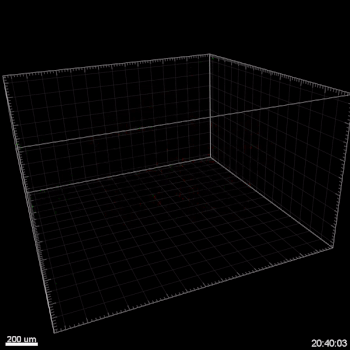
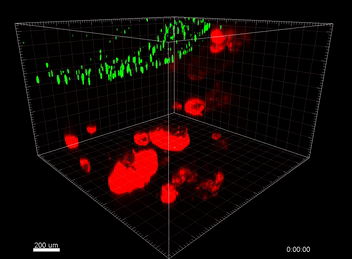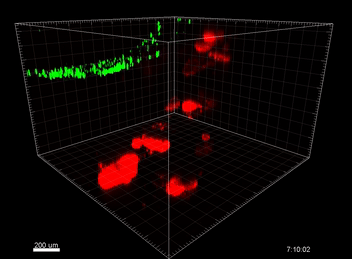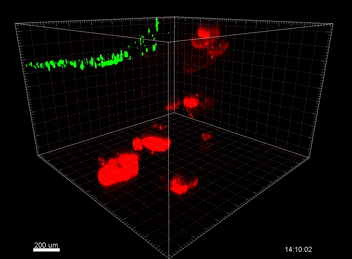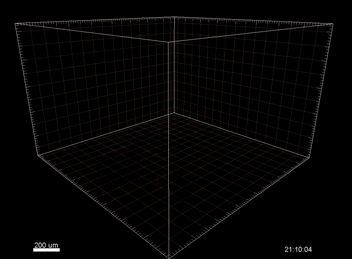
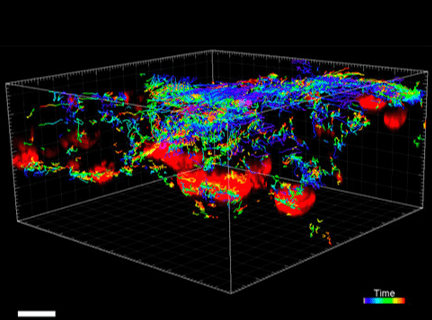
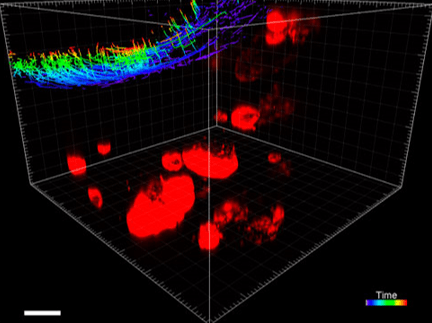
Figure 3. VitroGel® ORGANOID improves immune cell-epithelial interactions in a co-culture model of Human Gastric Organoids (HGO) and Dendritic Cells.
The videos show a 20 h timelapse of mCherry-expressing human gastric organoids on the GOFlowChip co-cultured with CellTracker Green-labeled Monocyte-derived dendritic cells for 20 h. Organoids are embedded in VitoGel® ORGANOID-3 (left) and Matrigel(right). Poor movement of MoDCs (green) when co-cultured with and HGOs (red) embedded in Matrigel. Improved migration of MoDCs (green) towards HGOs (red) embedded in VitroGel® ORGANOID-3.
(Video credit to Barkan Sidar, Michelle Cherne, Jim Wilking, and Diane Bimczok from Montana State University). Cherne et al. doi.org/10.3389/fphar.2021.707891
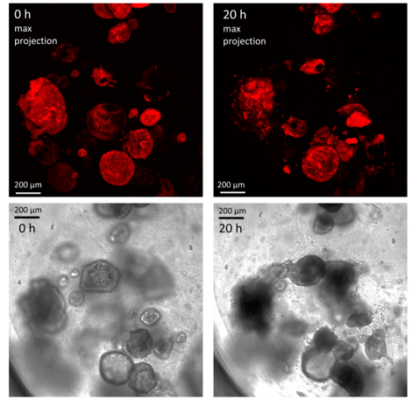
Figure 4. Human gastric organoids retain their circular morphology and size in V-ORG-3 for 20 h.
Human gastric organoids retain their circular morphology and size in V-ORG-3 for 20 h. Top panels: Representative confocal images of mCherry-expressing HGOs 0 h and 20 h after transfer from Matrigel to V-ORG-3, maximum Z-projection. Bottom panels: bright field images of the same HGOs shown in the top panels. Bar = 200 µm.
Cherne et al. doi.org/10.3389/fphar.2021.707891
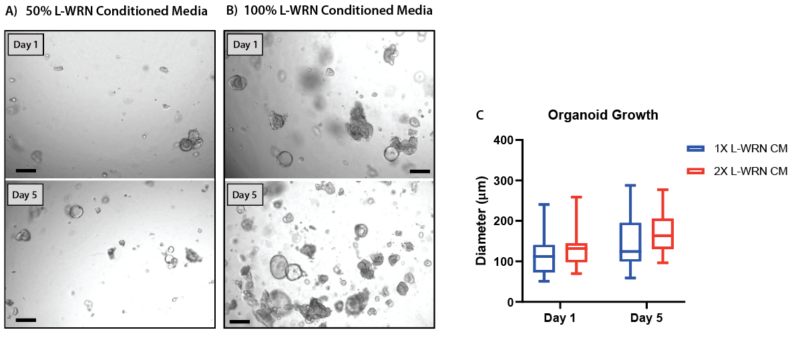
Figure 5. Increasing the concentration of L-WRN conditioned media improves organoid formation and growth in V-ORG-3.
Human gastric organoids (HGOs) were passaged by trypsinization then resuspended in gel using either (A) 50% L-WRN conditioned media (CM), the recommended growth factor concentrations or (B) whole L-WRN CM (100%), doubling the concentration of the organoid growth factors Wnt3a and Rspondin. HGOs were imaged one day after passaging (day 1) and five days later. (C) On day 1 and 5 the diameter of 20 organoids from each condition was compared. Box and whisker plot, unpaired t-test (P = 0.10).
Organoid 2D Hydrogel Coating Method
Figure 6. Organoids from breast cancer PDX cultured on VitroGel ORGANOID.
Cells were cultured on top of 2D hydrogel coating surface for 7 days.
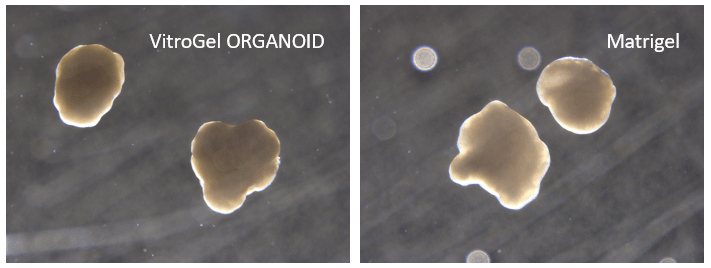
Figure 7. Cerebral organoid culture with VitroGel® ORGANOID and Matrigel comparison.
References/Publications
- Arthurs, A. L., Dietrich, B., Knöfler, M., Lushington, C. J., Thomas, P. Q., Fatwa Adikusuma, Williamson, J. M., Babikha, S., Tyla Damhuis, Jankovic-Karasoulos, T., Smith, M. D., Pringle, K. G., & Roberts, C. T. (2025). Genetically edited human placental organoids cast new light on the role of ACE2. Cell Death and Disease, 16(1). https://doi.org/10.1038/s41419-025-07400-x
- Nikeghbal, P., Burke, D., Armijo, D., Aldarondo-Quinones, S., Lidke, D. S., & Steinkamp, M. P. (2025). The Influence of Macrophages within the Tumor Microenvironment on Ovarian Cancer Growth and Response to Therapies. https://doi.org/10.1101/2025.01.29.635532
- Morelli, M., Lessi, F., Franceschi, S., Ferri, G., Giacomarra, M., Menicagli, M., Gambacciani, C., Pieri, F., Pasqualetti, F., Montemurro, N., Paolo Aretini, Orazio Santo Santonocito, Luisa, A., & Chiara Maria Mazzanti. (2024). Exploring Regorafenib Responsiveness and Uncovering Molecular Mechanisms in Recurrent Glioblastoma Tumors through Longitudinal In Vitro Sampling. Cells, 13(6), 487–487. https://doi.org/10.3390/cells13060487
- Costa, A. F., Senra, E., Campos, D., Faria-Ramos, I., Santos-Ferreira, L., Lamas, S., Gomes, J., Pinto, F., Teixeira, A., Abrantes, R., Duarte, H. O., Pacheco, M., Pinto, M. T., Maia, A. F., António Pombinho, Barros, R., Fernandes, V., Casanova-Gonçalves, F., Sousa, F., & Barbosa, J. (2024). Monensin as potential drug for treatment of SLeX-positive tumors. MedRxiv (Cold Spring Harbor Laboratory). https://doi.org/10.1101/2024.03.11.24304048
- Menjivar, N. G., Gad, A., Thompson, R. E., Meyers, M. A., Hollinshead, F. K., & Tesfaye, D. (2023). Bovine oviductal organoids: a multi-omics approach to capture the cellular and extracellular molecular response of the oviduct to heat stress. BMC Genomics, 24(1). https://doi.org/10.1186/s12864-023-09746-y
- Agostini, A., Guerriero, I., Piro, G., Quero, G., Roberto, L., Esposito, A., Caggiano, A., Priori, L., Scaglione, G., Francesco De Sanctis, Antonella Sistigu, Musella, M., Larghi, A., Gianenrico Rizzatti, Lucchetti, D., Alfieri, S., Sgambato, A., Bria, E., Bizzozero, L., & Arena, S. (2023). Talniflumate abrogates mucin immune suppressive barrier improving efficacy of gemcitabine and nab-paclitaxel treatment in pancreatic cancer. Journal of Translational Medicine, 21(1). https://doi.org/10.1186/s12967-023-04733-z
- Hakuno, S., Michiels, E., Kuhlemaijer, E., Rooman, I., Hawinkels, L., & Slingerland, M. (2022). Multicellular Modelling of Difficult-to-Treat Gastrointestinal Cancers: Current Possibilities and Challenges. International Journal of Molecular Sciences, 23(6), 3147. https://doi.org/10.3390/ijms23063147
- Powell K. Adding depth to cell culture. Science, 356(6333), 96–98. https://doi.org/10.1126/science.356.6333.96
Matrigel is a trademark of Corning Incorporated.
| Organoid Version | Discovery KIT, ORGANOID-1, ORGANOID-2, ORGANOID-3, ORGANOID-4 |
|---|---|
| Size | 4 x 2 mL, 2 mL, 10 mL |