Research Highlights
A One-Two-Three Punch Against Breast Cancer
Institutions:
Korea Advanced Institute of Science and Technology
Team:
Beha, M.J., Kim, J.-C., Im, S.H., Kim, Y., Yang, S., Lee, J., Nam, Y.R., Lee, H., Park, H.-S., & Chung, H.J.
Disease Model:
Breast Cancer
Hydrogel:
VitroGel® Hydrogel Matrix
Anticancer drugs were combined with CRISPR/Cas9 gene editing complex in a powerful nanoparticle structure that shows promise in targeting tumor cells and shrinking them in vivo.
The gene editing tool CRISPR/Cas9 has engendered a Noble Prize and a great deal of excitement surrounding its possibility use as a therapeutic agent against human diseases with a genetic component. However, to date, our ability to apply this powerful technique against the incredible complexity of cancer remains uncertain. The tumor microenvironment is constantly under change, and our capacity to bring gene editing with exquisite specificity to certain cells is proving to be daunting.
In this paper, a team of South Korean scientists hoped to take a slightly different approach with CRISPR/Cas9, that is, to combine gene editing with traditional chemotherapeutic drug delivery. The idea was to develop a self-delivered nanoplatform in which a drug could be bioconjugated to the CRISPR system, akin to how antibody-drug conjugates are able to use the specificity of Ab proteins to deliver their payloads with precision. In this case, the payload could potentially include both a drug and a gene editing functionality, a “double threat” of anticancer treatment.
The authors created such a nanoplatform by adding an azide-containing anti-cancer drug (azido-phenylalanine) to the Cas9 protein through “click” chemistry. This had been done before, but the researchers went further to create an “all in one” nanoplatform that included the Cas9 protein, the drug, guide RNAs, and a carrier polymer. All of these components self-assembled into a package that the authors dubbed “ComBiNE.” The package formed spherical structures that could be seen through electron microscopy.
With these ComBiNE particles, the authors proceeded to perform in vitro and in vivo tests of their ability to fight cancer cells. They first showed that ComBiNE packages could be used to target a specific gene in cell culture. Indeed, when the nanoparticles were asked to edit the RAD52 gene, one involved in DNA recombination, they could do so. This result was extended to a demonstration of a retardation of the growth of HCC1937 breast cancer cells with the BRCA-1 mutation in culture.
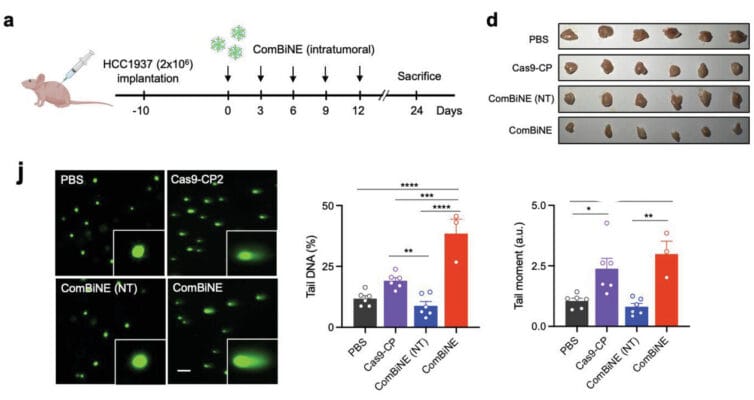
The authors finally turned to an in vivo mouse model in which the gene-editing prowess of ComBiNE might be able to target the editing of the RAD52 locus in live animals. By combining HCC1937 cells with a suspension of TheWell Bioscience’s VitroGel® Hydrogel Matrix, the authors injected these cells and created a xenograft in which breast cancer tumors were growing in a mouse. After 10 days of such growth, the authors then injected the ComBiNE complex (or controls) into the tumors and watched to see what would happen. Indeed, after 5 or 24 more days, the tumors that had received the full ComBiNE package (as opposed to just portions of it) demonstrated a statistically smaller size and growth rate.
In the end, this study opens up a new frontier for the potential use of gene editing tools to fight cancer. The combination of drug delivery, gene editing, and specific cell type targeting could be the magic mix that helps bring this powerful new technology to the forefront of oncology.