Research Highlights
Bringing a Small Intestine Model into the Laboratory
Institutions:
University of Graz, Austria
Team:
Zeiringer, S., Wiltschko, L., Glader, C., Reiser, M., Absenger-Novak, M., Frölich, E., & Roblegg, E.
Disease Model:
Intestinal Absorption
Hydrogel:
VitroGel® Hydrogel Matrix
Three hydrogels were tested as possible extracellular matrix mimics to establish a viable model of the human small intestine in the lab.
The extracellular matrix, or ECM, is a network of molecules that determines the mechanical strength and flexibility characteristics of the surrounding tissues. Being organized in a cell & tissue-specific manner, it is very important to emulate the in vivo ECM as closely as possible when constructing a model system in the laboratory that can reveal important biological functions. This is particularly true for models of intestinal systems, which need to reflect the accurate absorption and nutrient passage events that happen in real animals. The authors of this study held this ECM emulation as a prime concern when they compared three different types of commercial hydrogels that are commonly used in lab-based models of intestine function.
The authors, a group of Austrian cell biologists, sought to compare the properties of TheWell Biosciences’ VitroGel® hydrogel matrix with two alternative hydrogels, Matrigel and Peptigel, when used as a primary component of an in vitro human small intestinal model. Each of these hydrogels is comprised of a complex mixture of macromolecules, including collagen and laminin proteins, making them potential good mimics of both the interstitial matrix and the basement membrane, the two basic ECM types. However, they differ in their specific compositions and methods of preparation. When combined with the cell types that are commonly employed in a small intestinal mimicking system, such as enterocytes, mucus-producing goblet cells, and M cells, these ECM emulating hydrogels represent a very critical piece of the puzzle.
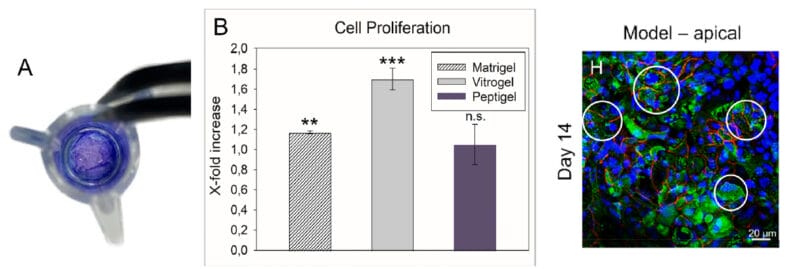
The authors cultivated various lines of endothelial cells (e.g., Caco-2 cells and HT29-MTX cells) in co-cultures in microtiter plates. The wells of the plate were coated with either 20 μL of Matrigel, or 40 μL of one of two synthetic alternative hydrogels, Peptigel or TheWell Biosciences’ VitroGel® 3D. After a 7-day or 14-day culturing process, the resulting ECM-emulating modules were characterized in terms of their rheological behavior and their impact on cell proliferation, including drug permeability, which is a fundamental characteristic of the small intestine. Notably, while all three hydrogels produced systems with similar viscosities and resiliencies to sheer forces, those that employed VitroGel showed a 1.7-fold greater propensity to promote cellular proliferation, compared to either Matrigel or Peptigel. This difference was statistically significant. Finally, after the authors analyzed how well drug-like substances of varying intrinsic permeabilities could be transported through these three systems, and they found that all of them behaved similarly to ex vivo systems in this regard.
In conclusion, the authors were successful in creating a viable model of the human small intestine that could be extremely valuable for drug permeability assays prior to the need for full-on animal testing. All the hydrogels that they used to provide an ECM mimic were viable in this regard, although VitroGel was clearly the most potent in promoting cellular propagation, making it the hydrogel of choice for future such models.