Research Highlights
COPD-Linked Extracellular Vesicles Transfer HIF-1α, Fueling Lung Cancer
Institutions:
Fondazione IRCCS Istituto Nazionale dei Tumori, Milano, Italy
Team:
Petraroia, I., Ghidotti, P., Bertolini, G., Pontis, F., Roz, L., Balsamo, M., Suatoni, P., Pastorino, U., Ferretti, A.M., Sozzi, G., & Fortunato, O.
Disease Model:
Chronic Obstructive Pulmonary Disease (COPD)
Hydrogel:
VitroGel® 3D
A definitive link between COPD and lung cancer was identified by determining that extracellular vesicles from COPD patients transfers a particular factor, HIF-1α, to epithelial cells, thereby triggering tumorigenesis.
Chronic obstructive pulmonary disease, or COPD, is a common result of cigarette smoking and is a clear risk factor for the development of lung cancer. Both COPD and lung cancer are known to be triggered by, or accelerated by, the activation of specific inflammatory pathways in the cell. The cell surface marker CD133 is one that has been implicated in the progression down the pathway from stem cells to non-small-cell lung cancer (NSCLC), but the relationship between CD133+ cells and COPD had not been established, nor had the exact concurring cellular events that occur in both COPD and NSCLC.
In this paper, the author compared the cellular characteristics of two populations of volunteer heavy smokers to get at these relationships. One group had COPD while the other did not. The specific goal was to evaluate the events leading to the release of the contents of extracellular vesicles (EVs), which in many cases triggers the epithelial-to-mesenchymal transition (EMT), a hallmark of tumorigenesis.
The team of cell biologists from The Italian National Tumor Institute obtained clinical samples of EVs isolated from the plasma of heavy smokers and from volunteers with COPD. The cellular origins of these EVs were estimated by an analysis of cell surface markers such as CD31. The authors also had for reference immortalized human bronchial epithelial cells (HBEC) of varying genotypes. With these two sets of materials, the team could determine the potential effect of circulating EVs on their propensities to induce cellular features that could trigger tumorigenesis.
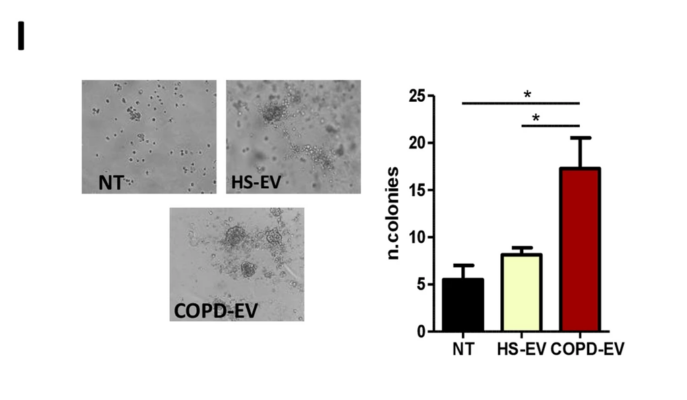
When the authors mixed the COPD-derived EVs and HBEC cells, they observed that the former definitely altered the cellular subpopulations of the latter. A particular HEBC genotype, KRAS-V12high, had been previously implicated as one that was commonly found in lung cancer. Upon interaction with COPD-derived EVs, this subpopulation had its fraction of CD133+ metastasis-inducing cells (MICs) driven up. Moreover, these cells were shown to be more aggressively growing through a 3D cell proliferation assay. The authors used TheWell Biosciences’ VitroGel® 3D to evaluate the ability of HBECs that had the KRAS-V12high genotype to grow as colonies. The authors determined that the likely ultimate cause of this triggering was the transfer of the hypoxia-inducible factor 1-alpha (HIF-1α) to the HEBCs, driving their MIC expansion and tendency to form tumors.
In the end, this set of experiments pointed a conclusive finger on the link between COPD and the propensity to develop lung cancer. The authors found that there were in fact significantly higher levels ofcHIF-1α in the EVs taken from patients who ultimately contracted lung cancer than from those who did not, despite the fact that both groups had COPD. But the bottom line is that the cellular events that transpire in the onset of COPD are conclusively forebearers of those that transpire in lung cancer, further cementing a causal relationship between these two debilitating disorders – and between each of them and cigarette smoking.


