Research Highlights
Restraint Upon Embryonic Metatarsal Ex Vivo Growth by Hydrogel Reveals Interaction Between Quasi-Static Load and the mTOR Pathway
Institution:
Royal Veterinary College, London
Team:
Caetano-Silva S., Simbi B.H., Marr N., Hibbert A., Allen S.P., and Pitsillides, A.A.
Application:
Creation of a cell culture environment that emulates a quasi-static load to test mechanical influences on limb growth.
Disease Model:
Mouse limb skeletal development
Hydrogel:
VitroGel® 3D
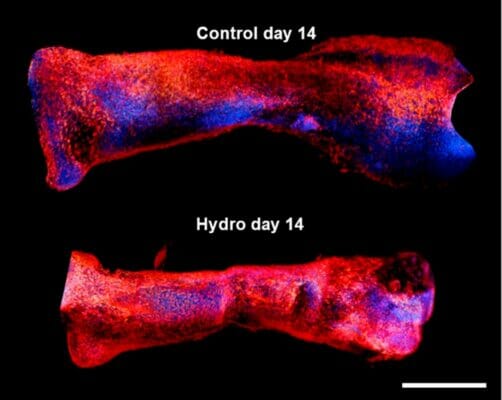
The factors that control skeletal limb development in mammals are only partially understood. Clearly there is some interplay between molecular and external forces that triggers or slows cell growth and ossification during ontogeny. To a first approximation, the Heuter–Volkmann law applies, in which static compression slows the longitudinal growth of embryonic cells, while static tensional forces accelerate it. This study attempted to deconvolute the various contributions of mechanical forces and gene expression pathways––particularly the mTOR pathway––that act upon early mesenchymal cells during development. The information gleaned in this regard could provide better models not only of comparative anatomy but also of how bones heal after being injured. Using a mechanical manipulation of murine metatarsi by encapsulating them in hydrogel, the authors of this work sought to observe the effects of hydrogel-induced quasi-static loading on cell growth. At the same time, the expression levels of a suite of molecular markers could be assayed in order to observe any important mechanical-molecular interactions.
A team of researchers from the Royal Veterinary College, London set out to test the hypothesis that the mTOR/NF-kβ pathways interact with mechanical loads to control the embryonic growth of mouse limb cells. They incubated murine embryonic metatarsals in various dilutions of VitroGel 3D, which they identified as a suitable hydrogel that could simulate the delivery of quasi-static mechanical loads. As a control, other metatarsals were not grown in hydrogel. The results showed that there was a clear impact of hydrogel environment on cell growth. Those cells maintained for two weeks in a 50% VitroGel 3D solution displayed an almost total arrest of longitudinal expansion. When the hydrogel was diluted 50-fold, the inhibition of growth dropped to the same level as the control, demonstrating that the hydrogel could impart a significant compressive strain. Furthermore, the team compared growth rates in the presence of factors known to be involved in the mTOR and NF-kβ pathways, namely leucine, rapamycin, betulinic acid, and SC-514. The involvement of these pathways was confirmed in that rapamycin and leucine significantly enhanced growth rates, albeit in different fashions. Finally, RT-PCR assays of gene products from 13 loci that previously have been implicated in bone growth were performed on both hydrogel-incubated and control metatarsi. Two loci, Col2 and Acan were found by a PCA to explain 72% of the total variance in growth restraint upon hydrogel incubation. These loci are linked to an S-G2/M cell cycle arrest, via extracellular matrix transcriptional changes. In summary, this study did find that VitroGel 3D can emulate quasi-static load environment suitable for the examination of mechanical forces that affect cell growth and differentiation. Moreover, the results supported the authors’ hypothesis of a complex interaction among molecular and mechanical factors for the modulation of limb growth.
Read the publication:
Related Products:


