Research Highlights
Estrogen Induces Apoptosis of Breast Cancer Cells in Aromatase Inhibitor Resistant Tumors
Institutions:
1. Department of Cancer Biology, Beckman Research Institute of the City of Hope, 2. Kyushu University, 3. Integrative Genomics Core, Beckman Research Institute of the City of Hope, 4. Department of Medical Oncology and Therapeutic Research, City of Hope National Medical Center
Team:
Mori H1, Saeki K1, Chang G1, Wang J3, Wu X3, Hsu P1, Kanaya N1, Wang X1, Somlo G4, Nakamura M2, Bild A4, Chen S1
Application:
Determine the mechanisms and effects of estrogen treatment on aromatase inhibitor-resistant breast cancer tumors.
Disease Model:
Breast Cancer
Hydrogel:
VitroGel® RGD
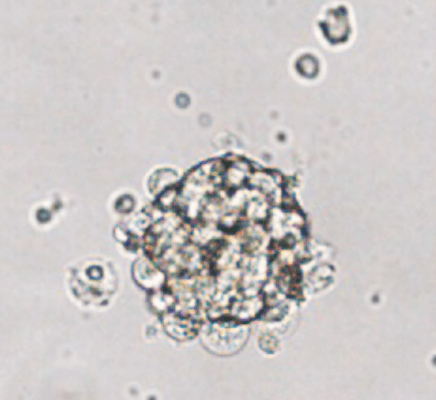
Hormone-dependent breast cancer uses estrogen to activate the estrogen receptor (ER). Aromatase inhibitors are used to treat this type of breast cancer because they suppress estrogen production. Although they improve outcomes for these breast cancer patients, resistance to this medication can eventually occur. Scientists created a cell line to mimic this by taking a hormone-dependent breast cancer cell line and depriving it of estrogen until resistance occurs. Patient-derived xenografts are more optimal than cell lines because they can preserve the multi-cellular system and some biological characteristics of the tumors. A xenograft from an aromatase inhibitor-resistant metastatic breast cancer tumor found in a brain was procured for this study, allowing them to study how estrogen could cause tumor regression in aromatase inhibitor-resistant breast cancer.
Organoids from the xenograft were embedded in VitroGel RGD hydrogel and received either estrogen or a placebo. Those treated with estrogen showed reduced proliferation in a dose-dependent manner, whereas proliferation continued with the placebo. Two different ER inhibitors were utilized to determine which ER was necessary for this effect. Estrogen no longer prevented tumor growth when the ERα-specific inhibitor called MPP was used. This means that estrogen acts on ERα, but not ERβ, to prevent growth in tumor organoids. Estrogen also decreased ERα cell density and protein content and ESR1 mRNA, which encodes ERα. Cells positive with Ki-67, a marker for breast cancer tumor cells, were also reduced with estrogen treatment. Protein related to cell cycle progression and proliferation,cyclin-B1 and mTOR, were also decreased after estrogen treatment. ER target genes, PGR, GREB1, and TFF1 increased while cell cycle progression genes, CDK1, TOP2A, E2F2, and MKI67, decreased in estrogen-treated tumor organoids. Moreover, two tumor suppressor genes, IL-24 and GADD45A, increased in organoids treated with estrogen. These experiments suggest that growth inhibition by estrogen is associated with reduced ERα cells, an increase in their target genes, cell cycle arrest, and growth suppression.
IL-24, a tumor suppressor cytokine that induces apoptosis in many cancer cell types, was substantially increased with estrogen treatment; the number of IL-24+ cells after estrogen treatment was double that of placebo. However, intermittent estrogen treatment (three rounds every 28 days) induced estrogen independence in tumor organoids. The number of ERα+ and Ki-67+ cells reversed by the intermittent treatment’s end compared to continuous treatment. Intermittent estrogen resulted in 60% of genes trending the same way as continuous treatment, with 40% trending similar to the placebo-treated group. All of the findings using organoids from a breast cancer tumor embedded in VitroGel suggest that estrogen can suppress tumor growth and eventually induce apoptosis of breast cancer tumor cells by acting on ERα.
Read the publication:
Related Products: