Research Highlights
Fishing for Therapies for Eye Cancer – Uveal Melanoma
Institutions:
Leiden University, The Netherlands; University of Liverpool, UK; & Friedrich-Alexander-Universität, Germany
Team:
Yin, J., Zhao, G., Kalirai, H., Coupland, S.-E., Jochemsen, A.G., Forn-Cuní, G., Wierenga, A.P.A., Jager, M.J., Snaar-Jagalska, B.E., and Groenewoud, A.
Disease Model:
Uveal Melanoma
Hydrogel:
VitroGel® Hydrogel Matrix
Zebrafish embryos were tested as a viable model to perform drug screening for anti-cancer drugs in cases where a mouse model would not be practical. The results showed remarkable promise to use zebrafish to support a PDX system to fight a recalcitrant form of eye cancer.
A particularly lethal cancer is uveal melanoma (UM), a rare form of eye cancer with a high malignancy rate. Although rare, the disease causes death in about 50% of cases, and there is currently no efficacious therapeutic strategy. Because UM is the most common form of eye cancer and has this unfortunate characteristic, there is clearly a need to develop a drug treatment model that can help researchers find effective approaches to combat its progression.
In this paper, a multinational team of oncologists and ophthalmologists sought to develop a zebrafish-based model system that could sustain patient-derived tissues as organoids long enough for drug screening. The PDX (patient-derived xenograft) concept is that tissues removed from a tumor can be propagated in an animal system in which a panel of anti-cancer therapies can be tried in a scenario that is specific and tailored to the particular genotype that an individual patient possesses; in other words, PDX is a form of personalized medicine. While mice are typically used to support the growth of tumor-derived tissue, other animals can provide advantages in terms of reduced cost, increased growth speed, and higher throughput for drug screening. In the current study, the short-lived nature of the UM tissues heightened this urgency. Thus the researchers here turned to zebrafish (Danio rerio), an organism that develops quickly and for which a large body of cell biology literature exists.
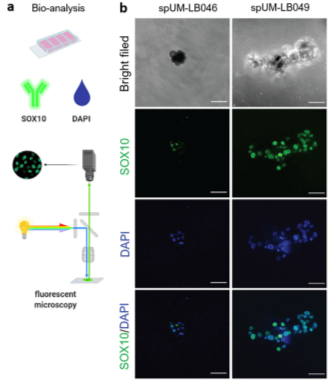
The scientists obtained tissue from cryobanks derived from UM patients (with consent) and grew the cells in traditional 2D culture. This process established the growth of organoids, which are spheroidal clumps of cells that exhibit many of the properties of in vivo tissues. To determine whether these organoids truly retained their UM characteristics, including their provenance as melanoma cells, the organoids were then mixed with TheWell Bioscience’s Vitrogel® Hydrogel Matrix and placed on a microscope slide for immunohistochemical analysis. After washing, the organoids were stained with anti-melan-A antibodies and secondary antibodies that would be indicative of a UM phenotype. Finally, fluorescently labeled cells derived from these organoids were injected into zebrafish, and the existence of a metastatic phenotype was assayed.
Using the histochemical staining, the researchers positively identified that the spheroids could maintain their UM-like characteristics in culture for at least seven days. This was encouraging for the continuation of the xenograft experiments in zebrafish to see if this species could support a viable anti-UM drug testing model. Fluorescence microscopic analyses did in fact allow the researchers to see that UM cells could be seen proliferating in zebrafish embryos six days after injection. The team thus concluded that the specifically UM-labeled cells derived from the primary patient spheroids maintained their metastatic potential. Finally, they applied the model zebrafish to drug therapy using known antineoplastic drugs for UM, including navitoclax, a small molecule that targets the apoptotic receptors of B-cell lymphomas. They found that the zebrafish embryos could tolerate the drugs well and that they reduced the tumor burden by approximately one-third across all the trial conditions.
The findings in this paper establish zebrafish as a potent model system in which drug screening for tumors that otherwise would not be amenable to a PDX approach can be possible. Thus, we have a potentially significant breakthrough in our race to develop effective treatments for lethal cancers such as uveal melanoma.
Read the publication:
Related Products: