Research Highlights
HPGDS in Adipose‑Derived MSCs Can Speed Wound Healing in Type 2 Diabetes
Institutions:
Peking Union Medical College, Beijing, China
Team:
Ouyang L., Qiu, D., Fu, X., Wu, A., Yang, P., Yang, Z., Wang, Q., Yan, L., and Xiao, R.
Application:
Wound Healing
Disease Model:
Type 2 Diabetes
Hydrogel:
VitroGel® RGD
Overexpression of hematopoietic prostaglandin D synthase (HPGDS) in the wound tissue of mice with type 2 diabetes can reduce inflammation and speed healing
Wounds in persons with Type 2 diabetes can often heal slowly. The complexities of this disease extend into myriad physiological arenas, including the events involved in re-vascularizing damaged tissue. Consequently, there has been much excitement surrounding the employment of mesenchymal stem cells (MSC) to accelerate wound healing in the case of diabetes. MSCs in other contexts have been shown to accompany the stimulation of several important growth factors, which in turn promote the creation of extracellular matrix (ECM) that is needed during tissue regeneration.
Unfortunately, there are challenges involved with MSC-based therapies. In particular, in diabetic individuals, inflammation tends to be persistent, and this increases the amounts of certain proteases that chew up the newly made ECM. The goal of the current study was three-fold. First, they wanted to generate a more complete gene expression profile to determine which proteins and RNAs were actually differentially expressed in the tissues of wounds from normal and type 2 diabetic mice. Second, they wanted to see if they could alter the rate of healing in mice using the results from their gene expression profile. And third, they wanted to create an in vitro model that could corroborate or extend their in vivo findings.
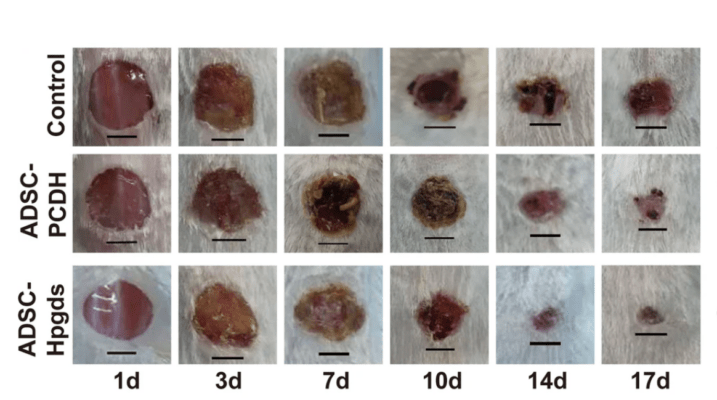
A team of scientists from Peking Union Medical College embarked on this set of goals. The gene expression profile revealed an interesting candidate: hematopoietic prostaglandin D synthase (HPGDS) appeared to be downregulated in diabetic mice compared to the normal controls. Normally this protein assists the healing process by recruiting neutrophils and CD8 T-cells, along with activating macrophages and stimulating the generation of growth factors. But in the diabetic environment, HPGDS was lacking, while inflammation was unchecked. For their in vivo studies, the authors prepared normal and HPGDS-overexpressed stem cells with TheWell Bioscience’s VitroGel 3D-RGD prior to injection into mice. A control group had only PBS injected. After a time course of 16 days, the authors checked the progress of the healing of induced wounds in these groups and found a correlation between HPGDS expression levels and the status of wound healing. Specifically, they noticed that HPGDS deficiency dramatically impeded wound healing. By contrast, cells that were upregulated in this study protein and seeded into VitroGel accelerated wound healing. Finally, their in vitro assay corroborated their live-animal data and supported their model of how the HPGDS protein promoted macrophage action and inflammation suppression.
While more studies are needed to bring these results into a clinical setting, the authors have demonstrated that HPGDS deficiency is a critical factor that impedes wound healing in type 2 diabetes. Overexpression of this protein could be exploited in the future to help those with this debilitating disease live healthier lives.
Read the publication:
Related Products: