Research Highlights
Advancing Borderline Ovarian Tumor Research: Precision Viability Assessment in Patient-Derived Organoid Models
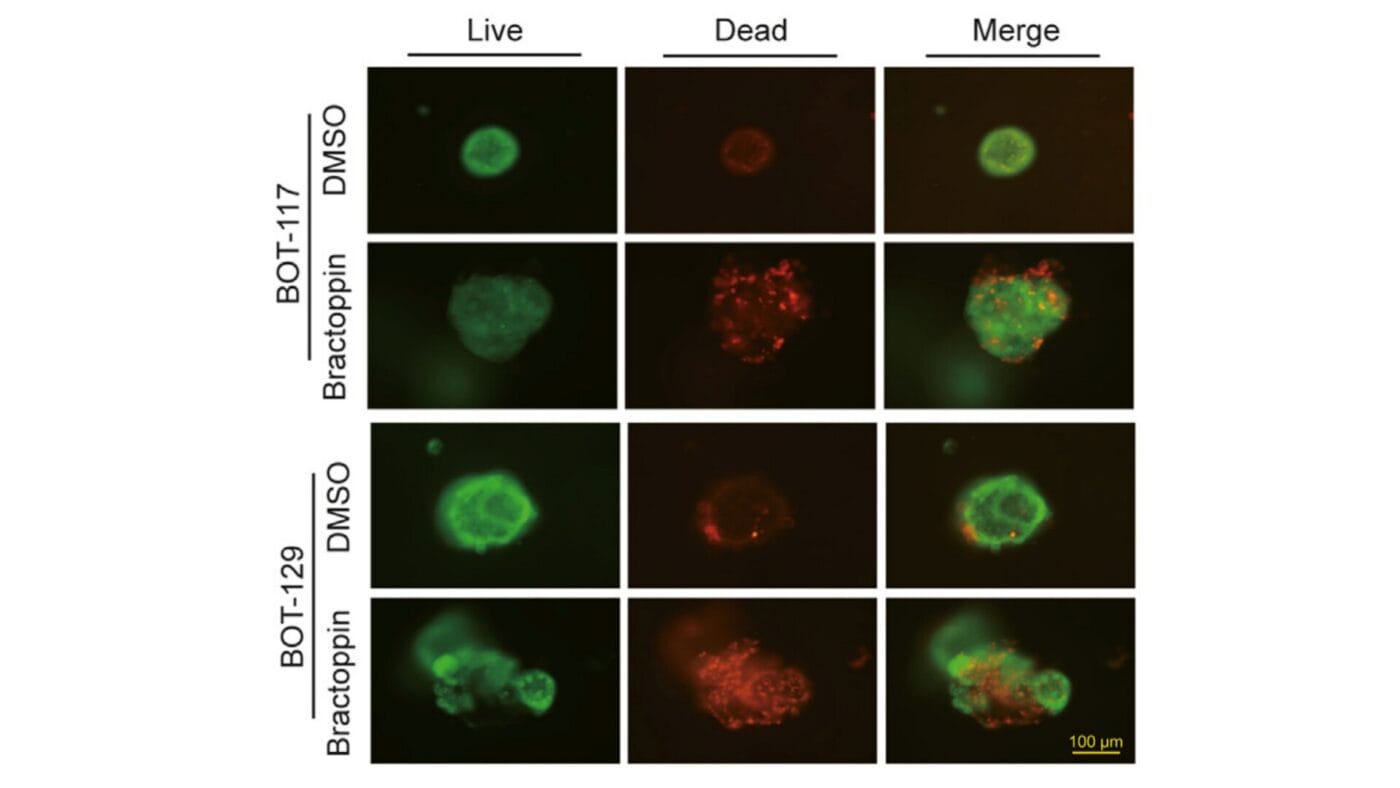
Cyto3D® Live-Dead Assay Kit enables the assessment of viability in patient-derived borderline ovarian tumor organoids for the evaluation of novel therapeutic compounds
Category:
Downstream Analysis
Subcategory/cell type:
Live-Dead Viability / Borderline ovarian tumor organoids
Institutions:
Department of Gynecology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, 210029, Jiangsu, China
Team:
Yicong Wan, Yashuang Zhang, Huangyang Meng , Huixian Miao, Yi Jiang, Lin Zhang, Wenjun Cheng
Biomarker
Cyto3D® Live-Dead Assay Kit (Cat. No: BM01)
The study explores the application of tumor organoid technology in borderline ovarian tumors (BOT), a category of tumors with limited treatment options and resistance to conventional chemotherapy. Researchers established patient-derived organoids (PDOs) to model BOT and evaluate potential therapeutic compounds, particularly Bractoppin, a BRCA1 carboxy-terminal domain (BRCT) inhibitor.
A critical aspect of the study was the assessment of organoid viability and response to treatment using the Live/Dead Assay Kit. This method enabled precise evaluation of cell survival and drug efficacy within the 3D culture system, providing valuable insights into the behavior of these tumor models under different conditions.
The findings demonstrate the successful establishment of long-term tumor organoids, providing a reliable model for studying borderline ovarian tumors and their response to targeted therapies. The Live/Dead Assay Kit played a crucial role in evaluating cell viability, allowing precise assessment of treatment efficacy within the 3D culture system. This approach ensures a more accurate representation of tumor behavior, improving the predictive value of drug screening.
By integrating viability assessment tools with advanced 3D culture systems, this study paves the way for more effective preclinical models. Future research could leverage this strategy to refine personalized treatment approaches and enhance the clinical relevance of organoid-based drug testing.





