Research Highlights
A New Valid Rhabdomyosarcoma Spheroid Culture Model for in Vitro Evaluation of Hypericin-Based Photodynamic Therapy
Institution:
University Hospital Marburg, Marburg, Germany
Team:
Hempfling L., Adamus A., Wagner B.R., Engel N., and Seitz, G.
Application:
Creation of a 3D cell culture scenario for the study of the efficacy of photodynamic therapy as an anti-cancer treatment.
Disease Model:
Pediatric alveolar rhabdomyosarcoma
Hydrogel:
VitroGel® RGD
Photodynamic therapy (PDT) has shown promise in the treatment of certain malignancies that are otherwise recalcitrant to chemotherapy and/or other anti-cancer protocols. The application of PDT is favored because it is minimally invasive and can be used either topically or systemically. Each PDT requires a photosensitizing agent, which releases reactive oxygen species such as singlet oxygen when stimulated with visible light. These species can damage or kill tumor cells in a localized fashion. This study focused on the efficacy of one particular photosensitizing agent, hypericin (HYP) which is a natural phytochemical derived from St. John’s wort. Previously HYP had been shown to be useful in the treatment of certain cancers. The goal was to examine the in vitro success of HYP to treat cultures of pediatric alveolar rhabdomyosarcoma (RMA) cells. While 2D cell cultures are often used as models for drug therapy, it has become apparent that 3D cultures, which are spheroids, better mimic in situ cancer cell populations that simulate cellular interactions, signal transduction, and gene expression. Thus, the current study aimed to establish a viable 3D cell culture model that was appropriate for RH-30 cells, which are an RMA cell line.
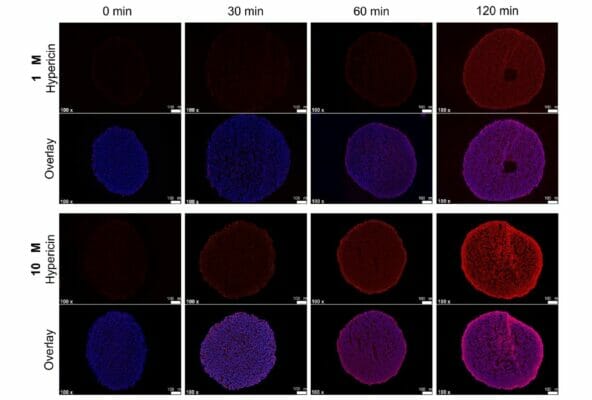
The University Hospital Marburg team of researchers created a variety of RH-30 multicellular spheroids using three scaffold-based and four scaffold-free techniques. These cells were embedded in VitroGel RGD using TheWell Bioscience’s established protocols. The architecture employed either a floating culture or a hanging drop, either in agarose-coated wells so that cell adherence was minimized. Various cell concentrations and preparations were then tested using PDT with HYP. The photosensitizer HYP was dissolved in DMSO to concentrations of either 1 μM or 10 μM, and then the spheroids were treated for two hours. Subsequently PDT was performed by illuminating the cultures with 200,000 lux of 30 mW/cm2 of white light from a brightfield microscope for varying times (5, 10, 15, and 20 minutes). Then interspheroidal HYP distributions were measured, and cell viability assays were performed to examine which treatment regime was the most efficacious. The results showed that HYP could penetrate the spheroids quite well in time- and concentration-dependent fashions, although the outer layers were more easily saturated. In terms of cell viability upon PDT, even the shortest light exposure time of 5 minutes resulted in significant cell death rates between 32–45% depending on HYP concentration. As expected, longer times resulted in lower cell viabilities, and the 10 μM HYP dosage generated somewhat lower rates than the 1 μM dosage. Even spheroids dosed with HYP but not treated with light showed decreased viability. Overall, this study was able to establish two simple, quick, and replicable 3D culturing methods (magnetic spheroid printing and orbital shaking) for the RMA cell line RH-30 that provide uniform spheroids. The research further revealed that exposure time, HYP concentration, and cell type (e.g., bladder cell-derived or colorectal cell-derived spheroids) all can have an effect on cell viability. The study successfully demonstrated the utility of 3D cell culture as an in vitro model that, while not perfect at emulating the in vivo scenario, can greatly facilitate the study of anti-cancer therapies.