Research Highlights
Shaking Up Tumors with Sound and Bubbles
Institutions:
Cheng Hsin General Hospital and National Taiwan University, Taiwan
Team:
Chen, K.-W., Hsu, P.-H., Huang, H.-L., Liu, H.-L., Lin, Y.-T., Hsu, C.-Y., Lin, J.-H., and Lin, Y.-H.
Disease Model:
Gastric Cancer
Hydrogel:
VitroGel® 3D
Antitumor drugs can be delivered to tumors with nanoparticles and microbubbles, and then the uptake of the drugs can be sparked by the application of ultrasound. This combination therapy works well against gastric tumors in a mouse model.
A nanoparticle, a small particle between 1 to 100 nm in size, has various valuable properties in science. One of these is their use in quantum dot technology to produce brilliant colors, but another is that they can be designed to bind to specific cells and deliver a payload of drugs to those cells. This feature makes them very promising in cancer research. Many anti-tumor therapeutic trials have proven efficacy by nanoparticle-based drug delivery. However, solid tumors are somewhat resistant to this approach, partly because their internal liquid pressure is so high that the drugs have difficulty penetrating the tissue.
The authors of this study, a Taiwanese team of oncologists, bioengineers, and pharmacists, hoped that ultrasound could help break up the tumors enough to help nanoparticle-delivered drugs be taken up in higher concentrations. They created a hybrid drug delivery module. This was a combination of negatively charged fucoidan (FC)-based nanoparticles with positively charged microbubbles of the inert gas C3F8. These microbubbles also help the module penetrate the tissues. The idea was that these combined tumor-seeking modules would recognize cancer cells by the characteristic cell-surface proteins they possess, such as P-selectin or CD44. These tend to be expressed to a high degree on the surfaces of breast, colon, gastric, and other cancer cells.
The plan by the authors was to deliver the anti-tumor drug doxorubicin (DOX) to gastric cancer cells in the laboratory using human gastric cancer cell lines and in vivo in a mouse model. They conjoined different formulations of DOX-containing nanoparticles to the microbubbles in various ratios and found an optimal balance of these components that should release the DOX properly at cellular pH values. They then cultured human gastric cancer cells in TheWell Bioscience’s VitroGel® 3D, which provided a fertile environment for cell growth and drug delivery assay. After mixing these cells with the optimized nanoparticle-microbubble-DOX modules, they exposed them to ultrasound for three minutes. Microscopic, Western blot and FACS analyses confirmed that the drug was released in this set-up and that ultrasound enhanced the amount of drug released by 3–10-fold compared to controls where no ultrasound was used. This combination of delivery module and ultrasound release is termed ultrasound-targeted microbubble destruction, or UTMD.
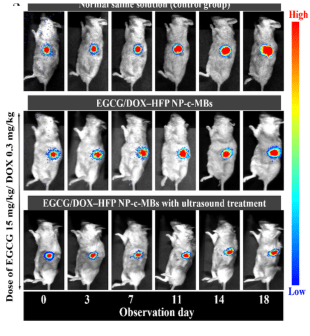
Next, the authors tested whether this drug delivery system could work in a live mouse model. They implanted gastric tumors into SCID mice and created solid tumors of at least 50 mm3. A set of injection experiments were initiated, with controls for no modules of any kind and no nanoparticles, while the experimental group received the DOX-loaded nanoparticle/microbubble delivery system. One additional advantage of using microbubbles is that they are readily visualized using traditional ultrasound imaging, so they can be located in the mouse’s body. UMTD pulses were applied to each group after 10 s, 130 s, and 300 s post-injection, and through this, the delivery could be confirmed. Moreover, after five injections across an 18-day time span, tumor growth was shown to be significantly reduced, the most so in the full experimental treatment using nanoparticles, microbubbles, and ultrasound application.
These results demonstrated the plausibility of combining four different tools to fight tumor growth, at least in the systems studied. Nanoparticle drug delivery, doxorubicin, microbubble conjugation, and ultrasound all appear to work together to enhance chemotherapy and speed up tumor death. Targeted drug therapy of particular tumor types has thus received additional hope.
Read the publication:
Related Products:


