Research Highlights
Validation of Low-Intensity Pulsed Ultrasonic Systems That Can Modulate the Doses That Are Delivered to Biological Targets
Institution:
The BioRobotics Institute, Pisa, Italy
Team:
Fontana F., Iberite F., Cafarelli A., Aliperta A., Baldi G., Gabusi E., Dolzani P., Cristo S., Lisignoli G., Pratellesi T., Dumont E., Jr., and Ricotti, L.
Application:
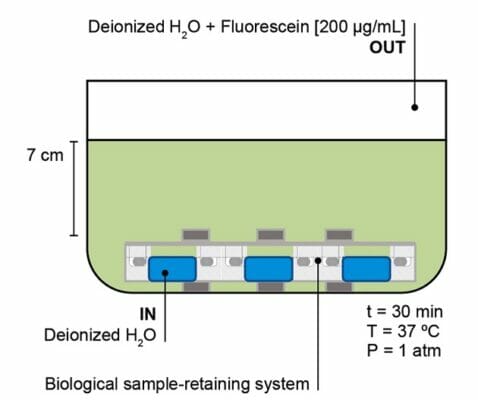
Addition of a microbiological growth environment to a low-intensity ultrasound chamber to test for external contamination.
Disease Model:
Ultrasound diagnostics
Hydrogel:
VitroGel® 3D
Ultrasound (US) is a non-invasive technique that has many diagnostic and therapeutic applications in medicine. It comes in two forms. The first is high-intensity, which can create a temperature rise in the target tissue that, in turn, can be used for ablation. The second is low-intensity. Applied at 0.5–3000 mW/cm2, this type of ultrasound does not cause a temperature rise in the tissue and can be used for tissue repair or regeneration. One application that US can be used for but which remains understudied is the precise control of dosages to a cellular target in vitro. In particular, low-intensity pulsed ultrasound (LIPUS) has demonstrated the ability to heal bone fractures and promote the growth of damaged nerves. Nevertheless, this technique has not been fully explored to modulate the exact dose that is delivered to a cellular system in vitro. There are several sources of error that preclude the delivery of a defined pulsed intensity to a specific surface area. The current study had the goal of designing and testing two LIPUS systems that could be used for an accurately controlled stimulation of cells.
LIPUS devices that could deliver either low frequency (38 kHz) or high frequency (500 kHz to 5 MHz) pulses to a defined target container were designed. Each employed a tank of water 35–50 cm high that held US transducers and a biological sample-retaining apparatus. These systems had to be constructed in such a way that highly controlled US wave transmission could take place that did not attenuate or reflect; these variations would prevent accurate and precise energy delivery to the target. Once constructed, a series of measurements were taken to analyze the acoustical properties of the system and the pressure homogeneity at the target. An important consideration, however, was the utility of the sample chambers to house and stabilize biological samples. Cell cultures had to be maintained at the target stage. Moreover, sterility was a concern, as these apparatuses need to be manipulated in myriad ways during operation, and samples need to be interchanged without the introduction of foreign microbiological material. Consequently, a VitroGel 3D hydrogel was prepared and applied to the film that served as the biological sample stage. These were left in the chambers for 1, 3, and 7 days and then removed and assayed for microbiological growth. This allowed design optimization of the systems. Although the water in the chamber could show bacterial contamination, the authors were able to alter the configuration of the sample-retaining apparatus to the point where no influx into the sample stage could be detected and thereby did not compromise the US experiments. Further tests demonstrated that the high- and low-frequency US pulses were not cytotoxic and that the use of LIPUS for in vitro cell stimulation could be validated.
Read the publication:
Related Products:


