White Papers
A Quick Organoid Viability Measurement by Using Cyto3D® Live-Dead Assay Kit
White Paper
Kalhara Menikdiwela Ph.D., John Huang Ph.D.
TheWell Bioscience Inc., Monmouth Junction, NJ 08852

Introduction
Organoids are three-dimensional (3D) tiny, self-organized tissue structures that mimic an organ’s functional and structural features. These self-organized 3D structures can be used to replicate much of the complexity of an organ. Compared to 2D monolayer cell cultures, organoids are becoming increasingly popular among research communities due to physiological similarities to intact tissues and the comparability of organoid-derived results to what we see in live organisms [1]. Thus, organoid cultures show great promise for in-depth studies in clinical and non-clinical areas to understand growth, development, cellular functions, and organ physiology.
Although organoid cultures are widely used and have improved tremendously in culture techniques and materials over the past few years, several areas, including cell viability testing, require further improvements. Identifying dead and live cells is one of the primary yet critical requirements in clinical and basic research, including drug discovery studies. Despite the availability of commercial kits to study cell viability, specifically in 2D monolayer cultures, researchers find it challenging to obtain accurate results with 3D organoids due to numerous limitations. These limitations include but are not limited to reduced penetration of reagents to the center of organoid/spheroid, inconsistency of staining from cell-to-cell within an organoid due to heterogeneity of cells, higher background signal, and false positive results from autofluorescence. Thus, a kit catering to provide effective solutions to overcome these limitations has become an essential requirement in 3D in vitro studies.
The Cyto3D® Live-Dead Assay Kit is a versatile kit that can overcome such limitations. It can be used not only with 2D cells but also for 3D cultures such as organoids in determining live/dead nucleated cells by using a quick one-step staining procedure. Further, Cyto3D® Live-Dead Assay Kit can work for both animal-based extracellular matrix (ECM) (e.g., Matrigel®) or synthetic hydrogel such as the VitroGel® system. The analysis can be performed using a simple dual-fluorescence system, which provides a quick and reliable outcome with its fast penetrable and consistent staining abilities.
Thus, we examined the use of Cyto3D® Live-Dead Assay Kit to recognize live-dead cells in mature and young intestinal organoids grown in a hydrogel-based extracellular matrix. We present the application of Cyto3D® Live-Dead Assay Kit to identify live-dead cells in 3D intestinal organoids using immunofluorescence image-based readouts.
Materials
Specimens: Intestinal Organoids
Hydrogels:
- VitroGel® ORGANOID-3 (Cat No: VHM04-3)
- Matrigel® Matrix
Biomarker: Cyto3D® Live-Dead Assay Kit (Cat No.: BM01)
Instrument: Fluorescence Microscope
Method
Intestinal organoids were thawed and suspended in both VitroGel® and Matrigel®. For VitroGel®, a suspension of organoids was prepared with 3X concentration of critical growth factors. VitroGel® ORGANOID-3 and organoid suspension were mixed at a 2:1 v/v ratio and kept for 15 minutes at room temperature for a soft gel formation. Similarly, for gel formation, organoids suspended in Matrigel® were incubated for 15 minutes at 37°C. Once ready, organoids were treated with 1X organoids culture medium supplemented with essential growth factors/inhibitors to stimulate organoid growth. Two sets of intestinal organoids were grown in 3D for 2 days and 5 days, respectively, in 5% CO2 and at 37°C supplemented with essential growth factors/inhibitors to stimulate organoid growth. The following steps were implemented in performing cell viability test in intestinal organoids using Cyto3D® Live-Dead Assay Kit:
Protocol for Cell Viability Analysis
- Bring the Cyto3D® Live-Dead Assay Kit to room temperature.
- Add 2 µL of Cyto3D® reagent to every 100 µL total volume in a well.
(Note: Adjust the volume of Cyto3D® reagent according to the total hydrogel volume and medium. For example, for 3D cell culture, 50 µL hydrogel + 50 µL cover medium = total volume of 100 µL).
- Incubate the cells at 37°C for 10 minutes. The cells are ready for cell viability detection.
Results and Discussion
Ever since the landmark study conducted by Sato et al. in 2009 to generate the first successful establishment of 3D organoid cultures [2], many research groups have successfully developed numerous organoid models using different organs and tissues, including intestine/gut [3], liver [4], brain [5], as well as certain malignant cancer/tumors organoids [6]. Although the innovative approaches and improvements in the past decade have assisted organoids in becoming a preferable model for numerous biomedical research studies, there is more room for improvements in generating reliable final outcomes. One such area is cell viability testing in 3D cultures.
Cyto3D® Live-Dead Assay Kit is a fast, ready-to-use, one-step staining procedure that can effectively study dead-live cells in 3D cultures, including organoids. Cyto3D® Live-Dead Assay Kit uses acridine orange (AO) and propidium iodide (PI) to label nucleated cells. AO stains all nucleated cells to generate green fluorescence. As indicated in Figure 1B, a two-day-old young intestinal organoid is mostly stained green, indicating a higher percentage of living cells than dead cells (Figure 1B, 1C & 1D). Meanwhile, PI only penetrates the membranes of nucleated cells with compromised membranes and stains the dead cells to generate red fluorescence. As visible in Figures 2C & 2D, dead-red stained cells have accumulated in the lumen of mature organoids (Figure 2). Thus, Cyto3D® Live-Dead Assay Kit effectively distinguishes live and dead cells in 3D intestinal organoids with minimum background and autofluorescence effects (Figures 1 & 2).
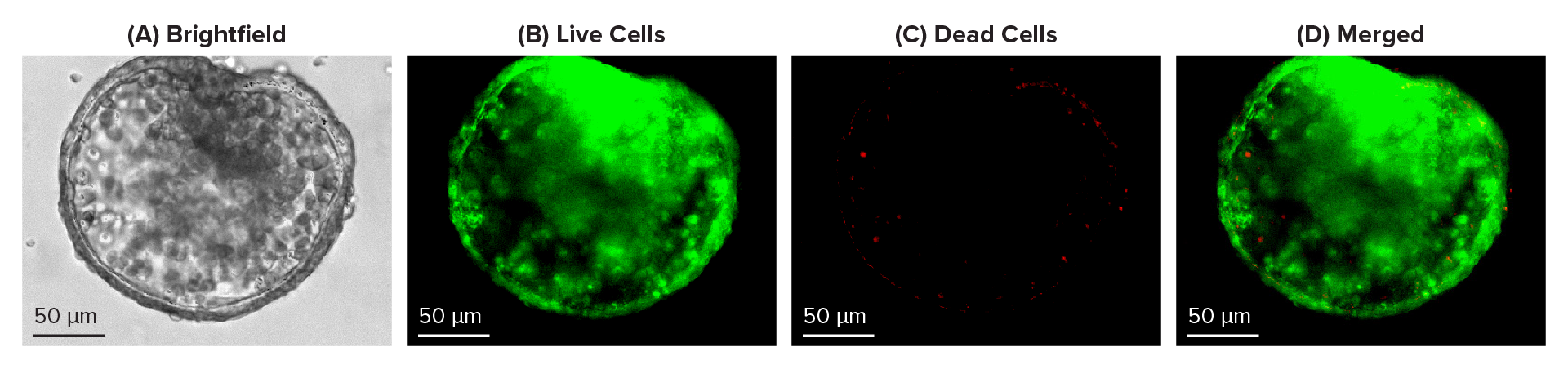
Figure 1: Live-dead cell viability images: Intestinal organoids stained with Cyto3D® Live-Dead Assay Kit. Intestinal organoids were cultured in regulated conditions for 2-3 days. Six microliters of Cyto3D® Live-Dead Assay reagent were mixed with organoid culture media (each well includes 150 µL of organoid culture media and 150 µL of hydrogel volume). The mixture was incubated at 37°C for 10-15 min, and the cells were observed under a fluorescence microscope. (A) A brightfield image of a young healthy intestinal organoid. Images show live cells (B: Green) and dead cells (C: Red) in a healthy intestinal organoid.
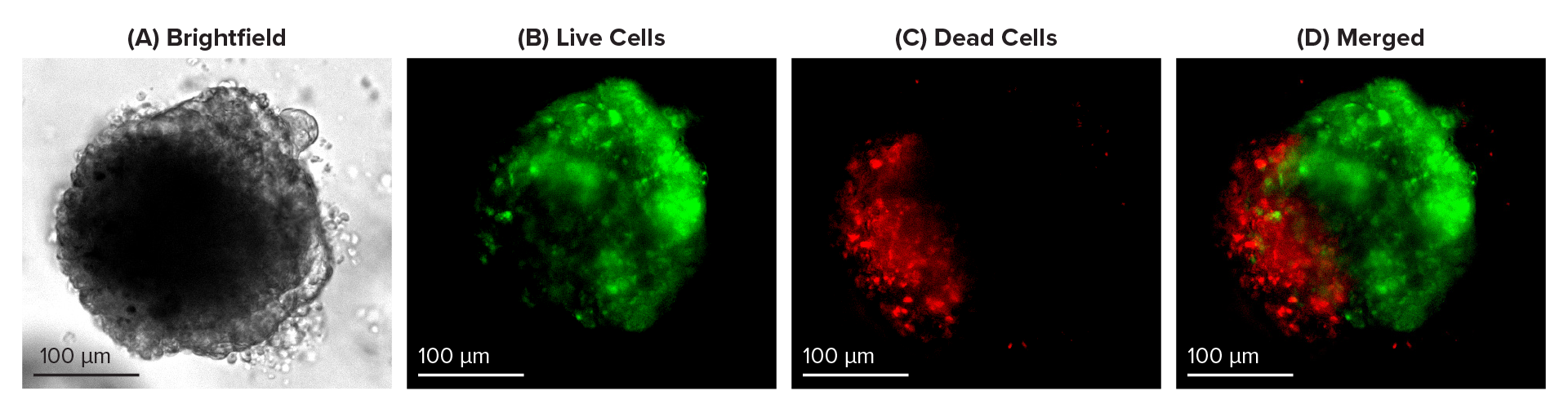
Figure 2: Live-dead cell viability images: Intestinal organoids stained with Cyto3D® Live-Dead Assay Kit. Intestinal organoids were cultured in regulated conditions for 5 days. Six microliters of Cyto3D® reagent were mixed with organoid culture media (each well includes 150 µL of organoid culture media and 150 µL of hydrogel volume). The mixture was incubated at 37°C for 10-15 min, and the cells were observed under a fluorescence microscope. (A) A bright field image of a mature intestinal organoid. Images show live cells (B: Green) and dead cells (C: Red) in a mature intestinal organoid.
Corroborating our findings, in the recent past, several research groups have used Cyto3D® Live-Dead Assay Kit to evaluate cell viability in their 3D in vitro studies [7-10]. Cheng W. and colleagues explored the involvement of Y-box binding protein 1 (YBX1) in alleviating platinum-induced stress using ovarian cancer patient-derived organoids (PDOs) [7]. Using Cyto3D® Live-Dead Assay Kit, they effectively determined live-dead cells in ovarian cancer PDOs against cisplatin treatment across different organoid lines (Figure 3A) [7]. They further studied carboplatin-cancer drug resistance in ovarian cancer-derived organoids that demonstrated an increased apoptotic cell population (labeled with Cyto3D® Live-Dead Assay Kit) with increased miR-1287-5p levels (Figure 3B) [8]. A drug discovery study conducted by Miao H et al. used Cyto3D® Live-Dead Assay Kit to identify live-dead cells in 3D PDOs in identifying novel roles of ferroptosis suppressor protein 1 (FSP1) on DNA damage repair in ovarian cancer PDOs (Figure 3C) [9]. Another cancer study by Markus Morkel used Cyto3D® Live-Dead Assay Kit to determine cell viability in colorectal cancer tissue-derived PDOs [10].
Such studies possess a huge promise for future therapeutic productions. One of the main limitations associated with cell viability kits used in 3D cultures is the uneven staining within or across wells. The hydrogel produced background staining due to autofluorescence when such kits were used. This directly affects the outcome and the image quality, limiting their use in 3D in vitro studies. However, Cyto3D® Live-Dead Assay Kit evenly stains the tissues/organoids cultured in 3D with no background signal.
Additionally, the compounds used in Cyto3D® Live-Dead Assay Kit can penetrate 3D structures better than the compounds used in other commercial kits, providing a better uniform staining result. These unique features of Cyto3D® Live-Dead Assay Kit make it a better kit for 3D cell viability testing than other commercial kits. Thus, the use of Cyto3D® Live-Dead Assay Kit in these discovery studies has contributed immensely to producing reliable and accurate results in terms of cell viability.
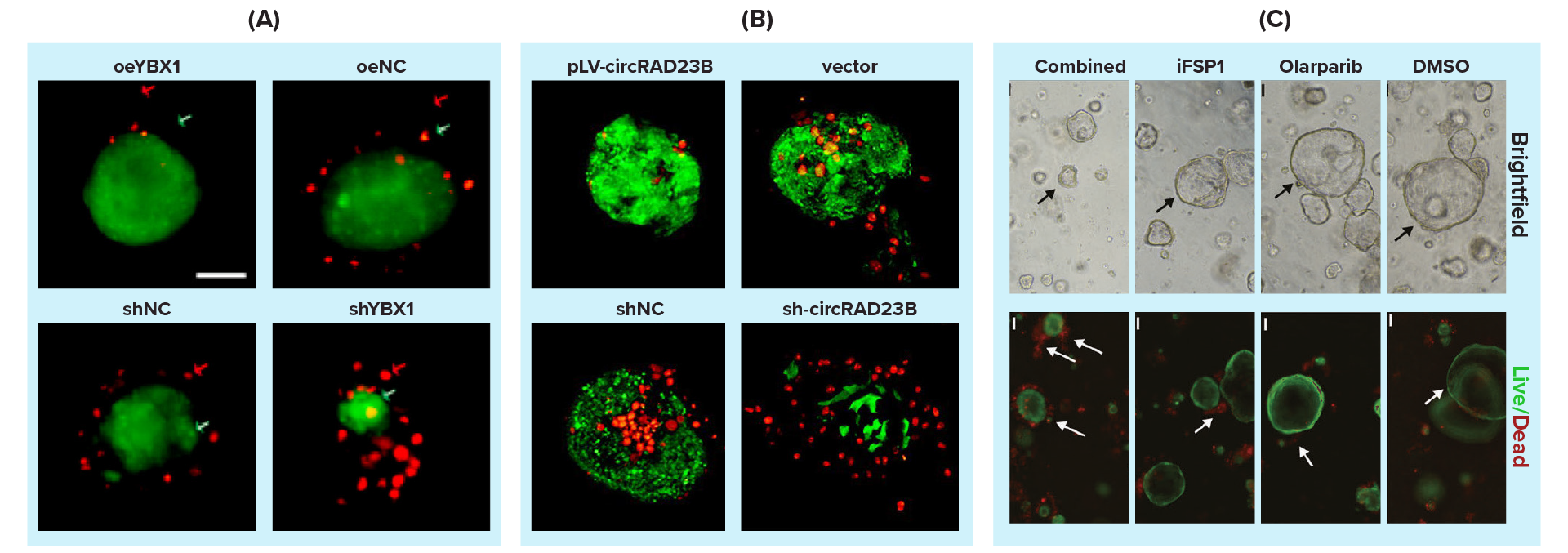
Figure 3: Use of Cyto3D® Live-Dead Assay Kit to test cell viability in 3D cultured Patient Derived Organoids (PDOs)/ovarian tumors. (A) High expression of YBX1 reduces sensitivity to platinum drugs in ovarian cancer organoids. After 48 hours of treatment with 30 μM cisplatin, various organoid lines (oeYBX1, oeNC, shNC & shYBX1) exhibited changes. The Cyto3D® reagent staining allowed the simultaneous detection of live and dead cells in organoid lines. (B) Functions of circRAD23B in patient-derived organoids (PDOs). Effects of 40 µM carboplatin pressure on PDOs for 48 hours and organoids stained with Cyto3D® reagent indicate cell viability/death; live cells are green, while dead cells are red. (C) The synergistic lethality of FSP1 inhibition and Olaparib in BRCA-proficient ovarian cancer (OC). Efficacy of PDOs in response to DMSO, Olaparib (20 μM), iFSP1 (25μM), and combination for 48 hours. In the live/dead assay, the green represented live cells, and the red represented dead cells (white arrows).
Conclusion
Cell viability can be effectively tested in 3D organoids grown in an extracellular matrix using Cyto3D® Live-Dead Assay Kit. The organoid’s maturity, age, or size did not impact the penetrating ability of the reagents of Cyto3D® Live-Dead Assay Kit, where the kit provided faster and clearer results, further emphasizing its reliability over many other commercially available cell viability test kits. The impact of the background signal and the autofluorescence was minimal or negligible. Thus, Cyto3D® Live-Dead Assay Kit can be invaluable, especially in 3D in vitro drug discovery studies, and will effectively contribute to the ongoing basic, pharmacological, and biomedical research by saving time and cost and providing more accurate and reliable results.
References
- Lancaster, M.A. and J.A. Knoblich, Organogenesis in a dish: modeling development and disease using organoid technologies. Science, 2014. 345(6194): p. 1247125.
- Sato, T., R.G. Vries, H.J. Snippert, M. Van De Wetering, N. Barker, D.E. Stange, J.H. Van Es, A. Abo, P. Kujala, and P.J. Peters, Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature, 2009. 459(7244): p. 262-265.
- Spence, J.R., C.N. Mayhew, S.A. Rankin, M.F. Kuhar, J.E. Vallance, K. Tolle, E.E. Hoskins, V.V. Kalinichenko, S.I. Wells, and A.M. Zorn, Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature, 2011. 470(7332): p. 105-109.
- Ang, L.T., A.K.Y. Tan, M.I. Autio, S.H. Goh, S.H. Choo, K.L. Lee, J. Tan, B. Pan, J.J.H. Lee, and J.J. Lum, A roadmap for human liver differentiation from pluripotent stem cells. Cell reports, 2018. 22(8): p. 2190-2205.
- Lancaster, M.A., M. Renner, C.-A. Martin, D. Wenzel, L.S. Bicknell, M.E. Hurles, T. Homfray, J.M. Penninger, A.P. Jackson, and J.A. Knoblich, Cerebral organoids model human brain development and microcephaly. Nature, 2013. 501(7467): p. 373-379.
- Broutier, L., G. Mastrogiovanni, M.M. Verstegen, H.E. Francies, L.M. Gavarró, C.R. Bradshaw, G.E. Allen, R. Arnes-Benito, O. Sidorova, and M.P. Gaspersz, Human primary liver cancer–derived organoid cultures for disease modeling and drug screening. Nature medicine, 2017. 23(12): p. 1424-1435.
- Meng, H., H. Miao, Y. Zhang, T. Chen, L. Yuan, Y. Wan, Y. Jiang, L. Zhang, and W. Cheng, YBX1 promotes homologous recombination and resistance to platinum-induced stress in ovarian cancer by recognizing m5C modification. Cancer Letters, 2024: p. 217064.
- Wang, H., Y. Zhang, H. Miao, T. Xu, X. Nie, and W. Cheng, CircRAD23B promotes proliferation and carboplatin resistance in ovarian cancer cell lines and organoids. Cancer Cell International, 2024. 24(1): p. 42.
- Miao, H., H. Meng, Y. Zhang, T. Chen, L. Zhang, and W. Cheng, FSP1 inhibition enhances olaparib sensitivity in BRCA-proficient ovarian cancer patients via a nonferroptosis mechanism. Cell Death & Differentiation, 2024. 31(4): p. 497-510.
- Uhlitz, F., P. Bischoff, S. Peidli, A. Sieber, A. Trinks, M. Lüthen, B. Obermayer, E. Blanc, Y. Ruchiy, and T. Sell, Mitogen‐activated protein kinase activity drives cell trajectories in colorectal cancer. EMBO molecular medicine, 2021. 13(10): p. e14123.
Key Takeaways:
- Label live-dead cells effectively in 3D tissue structures such as organoids and spheroids.
- Create uniform staining without background noise for clean, clear images with improved resolution.
- Implement a fast, one-step staining procedure that requires NO premixing to cover a broader range of applications in 3D and 2D cell cultures.