Advanced 3D Cell Models
Explant Model of the Normal Human Breast
Introduction
Figure 1: Structure of the Normal Human Breast Tissue Following 7 Days in this Culture System. Normal architecture of the human tissue, and the normal bilayer of luminal and basal epithelial cells is maintained.
Clinical studies of risk-reducing agents, such as tamoxifen, show a breast cancer risk reduction of at least 38%, and this can be maintained for up to 15 years (1, 2). However, uptake and adherence to this regimen are low due in part to a lack of efficacy and toxicity in some individuals (3, 4). The discovery of novel agents with the same or greater effect on risk and with minimal toxicity would enable a greater proportion of at-risk women to benefit from and tolerate preventative therapy. New prevention agents require extensive preclinical testing before being used in the clinic. Current patient-derived in vitro and in vivo breast models cannot recapitulate the full complexities of the human tissue, lacking human extracellular matrix, stromal, and immune cells (5-7). Our model of the normal human breast utilizes the tunable VitroGel® system (VitroGel® RGD, TheWell Bioscience, NJ, USA, TWG003-2), which maintains proliferation, structure (Figure 1), hormone receptors, T cells, and macrophages over 7 days (8). This model will allow us to perform preclinical testing of new risk-reducing strategies against breast cancer.
Materials
Media:
- DMEM/F12 containing B27 supplement (no vitamin A)
- 2 mM L-glutamine
- 100 U/mL Penicillin/100 µg/mL Streptomycin
Specimens:
Patient-derived Breast Tissue samples
Hydrogel:
VitroGel® RGD High Concentration Kit: VitroGel® RGD + VitroGel® Dilution Solution TYPE 2 (Cat#: TWG003-2)
Plasticware:
VitroPrime™ Cell Culture Inserts – 3 µm (Cat# VPE3-24-4)
Workflow
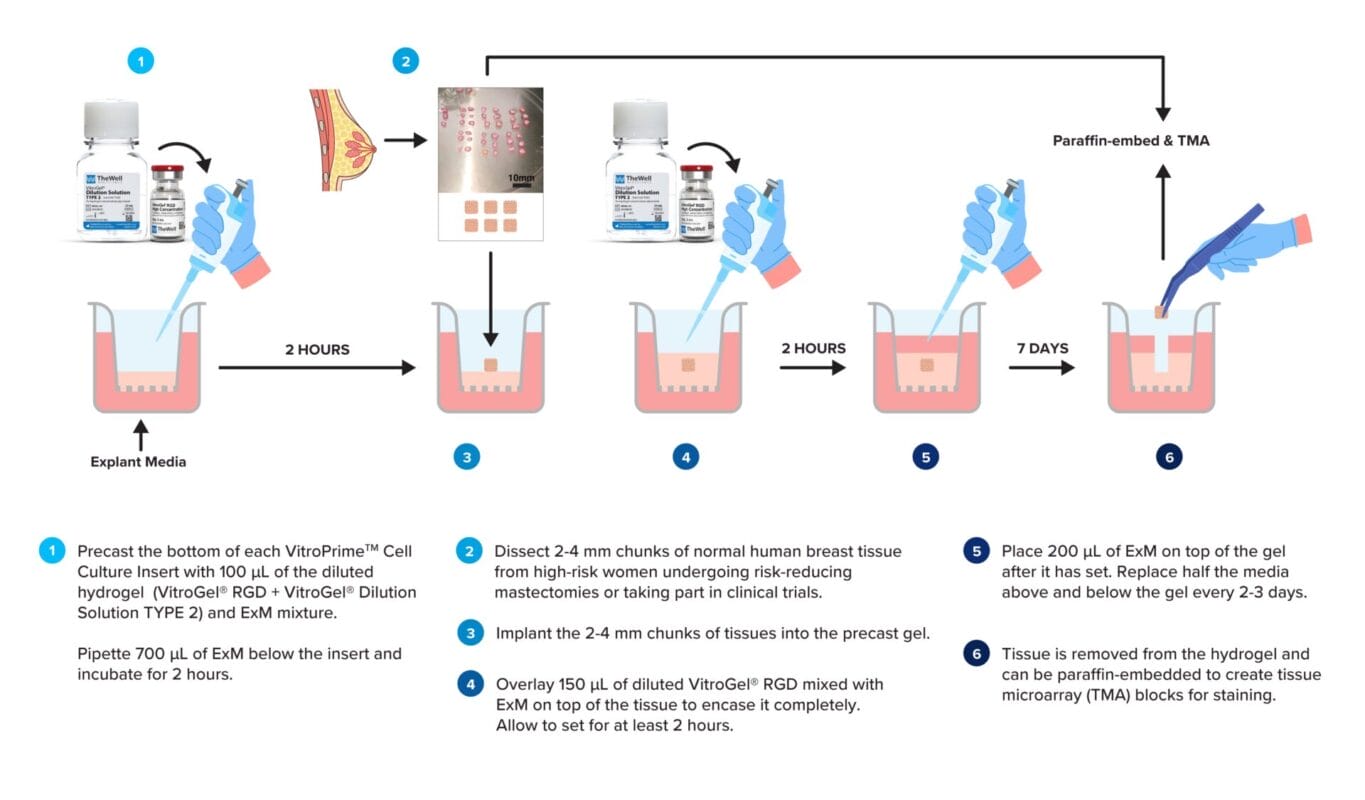
Figure 2: Graphical Workflow of Protocol.
Protocol
Casting the bottom layer of gel:
- Calculate the amount of diluted gel needed:
-
- Bottom: 100 µL (N+1)
- N = number of Cell Culture Inserts required
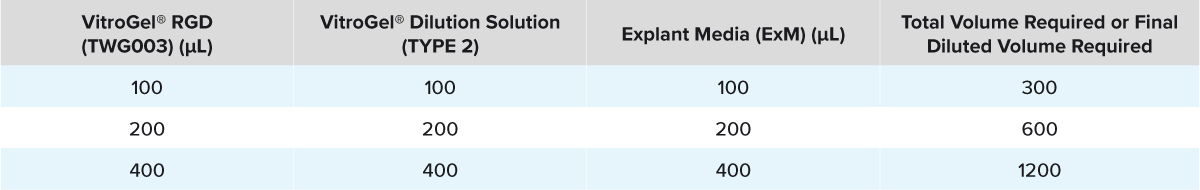
- Mix the desired volume of VitroGel® RGD and VitroGel® Dilution Solution (TYPE 2) in a suitable tube at a 1:1 ratio (v/v, Column 1 and 2 of Table 1).
- Add the required volume of ExM (Column 3 of Table 1) to the diluted VitroGel® RGD (Table 1) and pipette 100 µL of the diluted gel into the bottom of each VitroPrime™ Cell Culture Insert of the 24-well cell culture inserts.
- Note: Addition of the ExM will cause gelation to occur rapidly. To avoid waste, gels need to be mixed in batches.
- Pipette 700 µL of ExM into the well below the cell culture insert.
- Place this in an incubator at 37°C and 5% CO2 for 2 hours to set.
Table 1: Composition of the Diluted VitroGel® RGD
Preparing Human Breast Tissue:
- Using forceps and a scalpel, remove and dispose all the yellow fatty tissue as much as possible.
- Dissect the tissue into small 2-4 mm chunks of tissue using the same tools (Figure 3).
- Take the pre-cast hydrogel from the incubator. Place the dissected tissue into the insert on top of the hydrogel layer by using tweezers. Be careful not to damage the hydrogel layer.

Figure 3: Dissected Normal Human Breast Tissue. Tissue which has been dissected into small 2-4 mm chunks using forceps and a scalpel.
Casting the top layer of gel:
- Calculate the amount of diluted gel needed:
-
- Top: 150 µL (N+1)
- N = number of Cell Culture Inserts required
- Mix the desired volume of VitroGel® RGD and VitroGel® Dilution Solution (TYPE 2) in a suitable tube at a 1:1 ratio (v/v, Column 1 and 2 of Table 1.)
- Add the required volume of ExM (Column 3 of Table 1) to the diluted VitroGel® RGD (Table 1) and pipette 150 µL of the diluted gel on top of the tissue in the insert (Figure 4).
- Note: Addition of the ExM will cause gelation to occur rapidly. To avoid waste, gels need to be mixed in batches.
- Place this in an incubator at 37°C and 5% CO2 for 2 hours to set.
- Add 200 µL of ExM on top of the gel after setting.

Figure 4: Tissue Encased in VitroGel® RGD. Shows the dissected human tissue encased in VitroGel® RGD, achieved by casting the gel in two layers around the tissue.
Maintaining the Tissue:
- Replace half of the ExM from the top of hydrogel (replace 100 µL out of 200 µL) and half of the ExM from the outer well of the cell culture insert (replace 350 µL out of 700 µL) using a pipette with fresh ExM every 2-3 days. Be careful not to disrupt the hydrogel.
Removing the Tissue:
- Carefully remove the tissue from the gel using tweezers.
- Fix the tissue in 1 mL of 4% formalin for 24 hours at 4°C. The sample is read for other analysis.
Download the Protocol

Explant Model of the Normal Human Breast
This protocol was contributed by the Breast Biology Group from the Manchester Breast Centre, University of Manchester and modified by TheWell Bioscience.
Acknowledgement
Anthony J Wilby, Dr. Sacha J Howell, Dr. Gillian Farnie, and Dr. Hannah Harrison
This project has been funded by Prevent Breast Cancer.


Dr Sacha Howell

Dr Gillian Farnie

Breast Biology Group from the Manchester Breast Centre, University of Manchester
References
- Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827-34.
- Cuzick J, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16(1):67-75.
- Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention? NPJ Breast Cancer. 2017;3:20.
- Hackett J, Thorneloe R, Side L, Wolf M, Horne R, Cuzick J, et al. Uptake of breast cancer preventive therapy in the UK: results from a multicentre prospective survey and qualitative interviews. Breast Cancer Res Treat. 2018;170(3):633-40.
- Kenerson HL, Sullivan KM, Seo YD, Stadeli KM, Ussakli C, Yan X, et al. Tumor slice culture as a biologic surrogate of human cancer. Annals of translational medicine. 2020;8(4).
- Habel LA, Dignam JJ, Land SR, Salane M, Capra AM, Julian TB. Mammographic density and breast cancer after ductal carcinoma in situ. Journal of the National Cancer Institute. 2004;96(19):1467-72.
- Mohan SC, Lee T-Y, Giuliano AE, Cui X. Current status of breast organoid models. Frontiers in Bioengineering and Biotechnology. 2021;9:745943.
- Wilby AJ, Cabral S, Zoghi N, Howell SJ, Farnie G, Harrison H. A novel preclinical model of the normal human breast. Journal of Mammary Gland Biology and Neoplasia. 2024;29(1):9.