Research Highlights
Decoding New Insights into DCLK1’s Role in Cancer Cell Invasion and ECM Degradation
VitroGel® Hydrogel Matrix was used to establish mobility, gelatinase activity, protein-protein interaction, and invasion assays to unravel the underlying mechanisms behind the invasiveness of Head and Neck Squamous Cell Carcinoma (HNSCC).
Category:
3D Cell Models & Functional Assay
Subcategory/cell type:
Invasion/migration assays. Head and neck squamous cell carcinoma cells
Institutions:
University of Kansas Medical Center. Departments of Surgery, Biostatistics and Data Science, Otolaryngology and Cancer Biology.
Team:
Levi Arnold, Marion Yap, Laura Jackson, Michael Barry, Thuc Ly, Austin Morrison, Juan P. Gomez, Michael P. Washburn, David Standing, Nanda Kumar Yellapu, Linheng Li, Shahid Umar, Shrikant Anant, Sufi Mary Thomas.
Hydrogel:
VitroGel® Hydrogel Matrix (Cat. No: VHM01)
Head and neck squamous cell carcinoma (HNSCC) poses a significant global health challenge due to its high incidence rates and adverse effects on patient survival. A key feature of this malignancy is its propensity for locoregional invasion, which greatly contributes to patient mortality and treatment-related complications. The tumor cells’ ability to breach local extracellular matrix (ECM) barriers, invade critical structures such as the carotid artery, or metastasize to distant sites contributes to mortality. Understanding these early metastatic events is crucial for shedding light on HNSCC pathogenesis and identifying potential therapeutic targets in a largely underexplored aspect of HNSCC research.
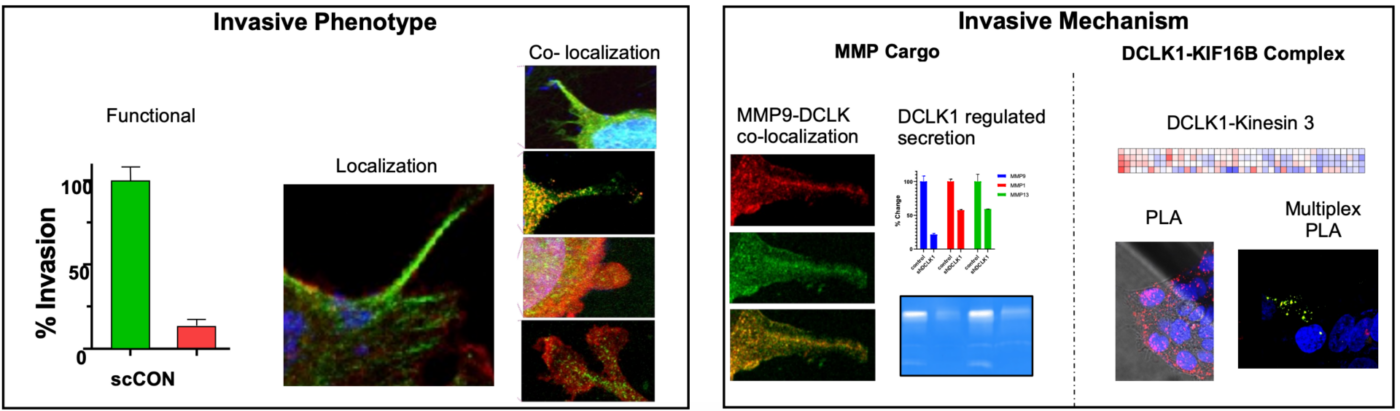
In this research, Arnold and team used VitroGel® Hydrogel Matrix to mimic the extracellular matrix (ECM) environment to understand cell mobility in a more physiologically relevant context. First, for invadopodia assays, VitroGel® was used to create a sandwich-like structure with cells between layers of ECM, facilitating the study of invadopodia formation and function. This setup helps observe how HNSCC cells interact with and degrade the ECM, providing insights into their invasive behavior. Furthermore, for assessing gelatinase activity, VitroGel® is used to evaluate the activity of these types of enzymes, like MMP2 and MMP9. By embedding Dye quenched (DQ)-gelatin in VitroGel®, measurements on how effectively these enzymes degrade the gelatin substrate can be performed, essential for understanding the role of DCLK1 in regulating MMP secretion and activity. In addition, VitroGel® was used in The Boyden chamber assay to quantify cellular invasion by measuring their ability to penetrate the gel. This is crucial for evaluating the impact of DCLK1 on cell invasion and understanding the molecular mechanisms involved. Finally, the authors used VitroGel® to perform proximity ligation assays (PLA), designed to detect and visualize protein-protein interactions at very close proximity, which is critical for understanding complex cellular processes and signaling pathways. Here, the PLA technique was utilized to identify the specific members of the Kinesin 3 family that are in proximity with DCLK1, demonstrating proximity between DCLK1 and KIF16B, an anterograde kinesin.
In summary, VitroGel® is vital for creating an in vitro model that closely mimics the ECM environment. This enables detailed studies of cell invasion, invadopodia formation, and enzyme activity in the context of HNSCC. Using VitroGel®, researchers can assess invadopodia formation and maturation in a controlled, ECM-like environment, providing clearer insights into their role in cancer invasion.