Challenges in Cancer Research and the Development of 3D Breast Cancer Models
As cancer therapies become more human-specific, researchers face growing challenges in accurately modeling disease progression and treatment responses.
- Limitations of Animal Models: Traditional animal models fail to replicate critical elements of the human tumor microenvironment (TME), limiting our understanding of cancer behavior.
- Inadequacy of Current In Vitro Models: Current in vitro models struggle to replicate the complexity of human tissue, hindering the testing of new anti-cancer drugs.
- Need for Improved Models: Despite early detection improvements, breast cancer rates continue to rise. Existing treatments like tamoxifen have not significantly lowered mortality rates, highlighting the urgent need for more accurate models that can recapitulate the full complexities of the human tissue.
VitroGel® Hydrogel Offers Versatile Applications for Advanced 3D Breast Cancer Studies
VitroGel® is an animal-free, ready-to-use, biofunctional hydrogel that mimics the ECM and supports cell growth with consistent results.
- Designed to closely mimic the ECM: Supports long-term tissue viability and maintaining key functions like epithelial proliferation and hormone receptor expression.
- Provides a stable and controlled microenvironment: Allows researchers to study cancer, stromal, and immune cell interactions, aiding in understanding therapy responses and drug resistance.
The Multiple Application and Studies
In Vitro 3D Model
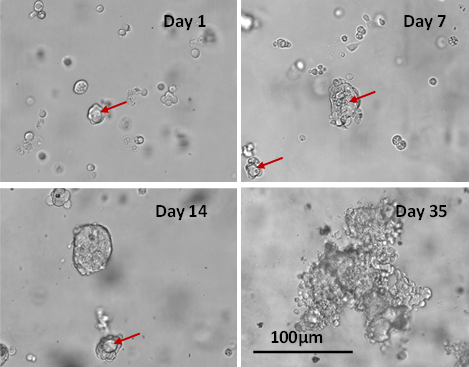
Advanced 3D Luminal Breast Cancer Model in VitroGel® System: Long-term 3D Cell Culture and Co-culture with Fibroblast Cells
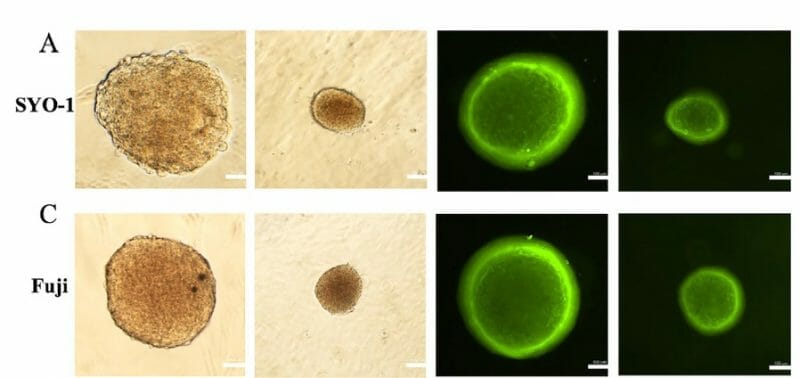
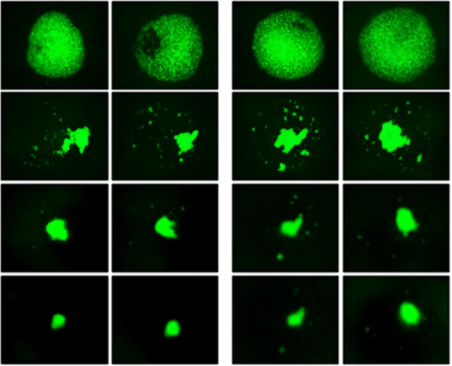
This study addresses the need for accurate 3D disease models by using VitroGel® Hydrogel Matrix, which supports long-term, multi-cellular cultures that mimic complex human microenvironments. Researchers developed a 3D luminal breast cancer model with MCF-7 cells and fibroblasts, demonstrating strong cell-matrix and cell-cell interactions. The method shows promise for creating comprehensive 3D co-culture systems across various cell types.

Ex Vivo/Co-Culture Models
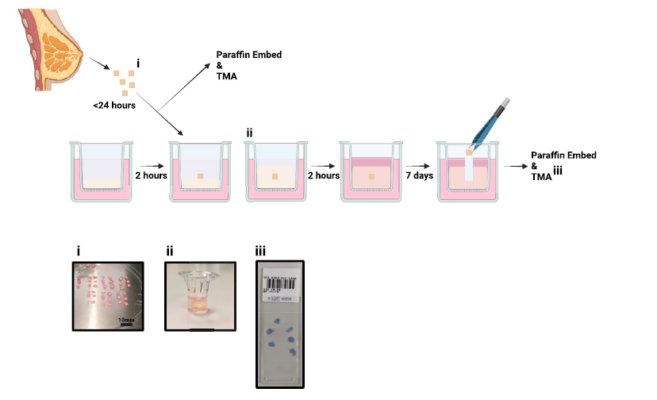
Explant Model of the Normal Human Breast
Clinical studies show that tamoxifen can reduce breast cancer risk by at least 38% for up to 15 years, but its use is limited by issues of efficacy and toxicity. The development of new, low-toxicity agents could help more women benefit from preventative therapy, though these require extensive preclinical testing. The authors present a breast model using the VitroGel® system, which maintains key tissue components and allows for testing new risk-reducing strategies.
In Vivo Models
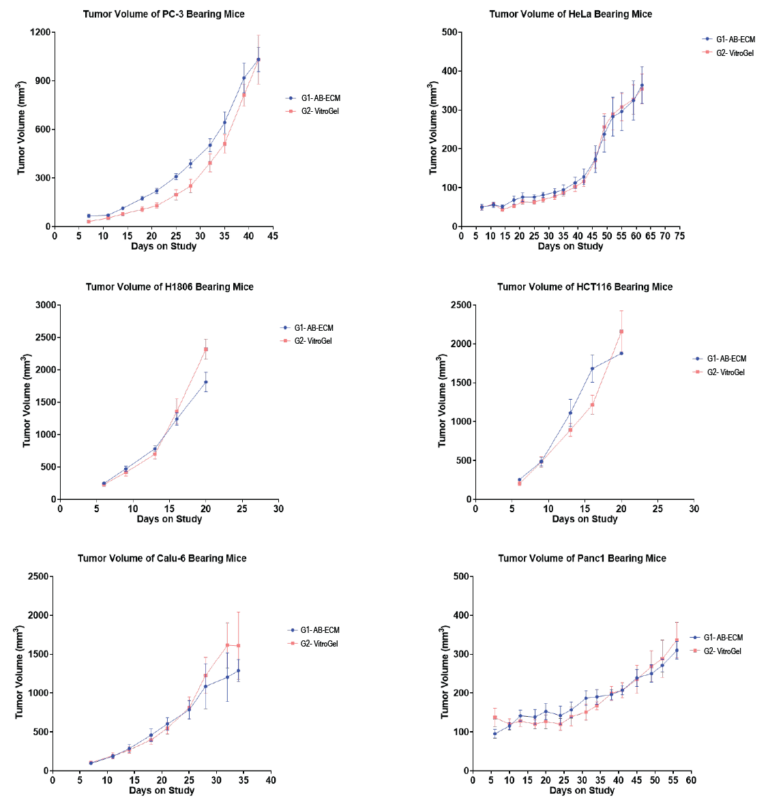
Cell Line-Derived Xenograft Using Xeno-Free VitroGel® System in Comparison to Animal-based Extracellular Matrix
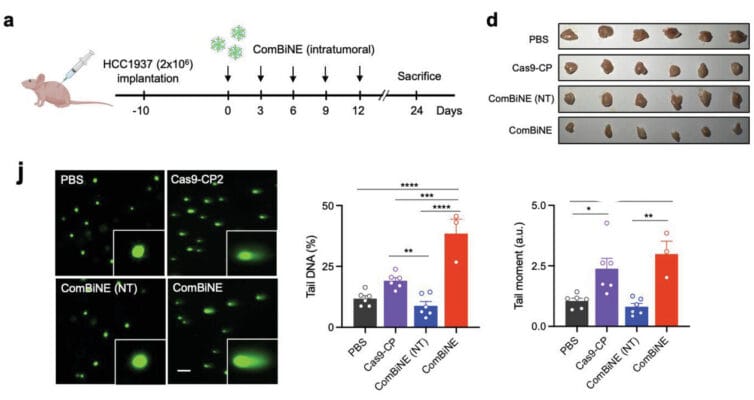
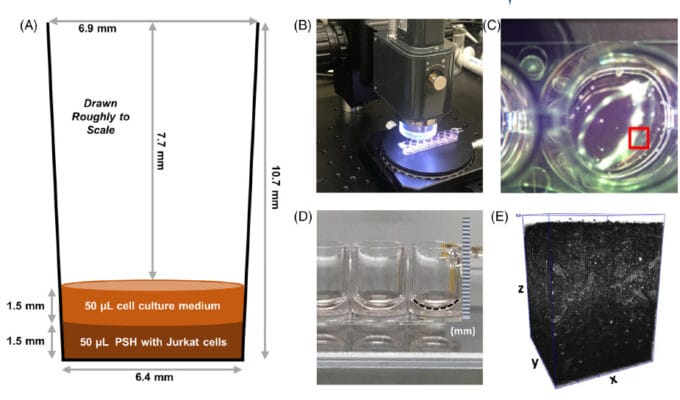
Cancer research faces challenges due to the individualized nature of disease progression, with models like Patient-Derived Xenografts (PDX) and Cell Line-Derived Xenografts (CDX) advancing the field. However, traditional animal-based extracellular matrices (AB-ECM) have limitations like batch inconsistency and slow production. This note compares VitroGel®, a synthetic alternative, to AB-ECM in CDX models, showing its potential to support tumor growth studies across various cancer cell lines more effectively.
Research Highlights
Product Highlights
VitroGel® Hydrogel Matrix (VHM01)
Ready-to-use, xeno-free hydrogel system that supports a wide range of cell types for different applications such as: in vitro 3D models, ex vivo/ co-culture, and in vivo models.
VitroGel® RGD
Tunable, xeno-free hydrogel system modified with cell adhesive peptide RGD to promote cell attachment and cell-matrix interactions during the 3D cell culture.
Other Tunable Hydrogels
Tunable hydrogels are available with various functional ligand modifications, providing a range of building blocks to create a customized microenvironment that closely mimics the natural extracellular matrix (ECM)
VitroGel® Organoid Recovery Solution
Non-enzymatic cell harvesting solution for quick and efficient recovery of 3D cells or organoids cultured with either VitroGel® hydrogels or an animal-based ECM.
Cyto3D® Live-Dead Assay Kit
Versatile live-dead assay kit for 3D and 2D Cell Culture, Organoids, Spheroids, Stem Cells, and Fluorescence Microscopy.
The VitroGel®-Based Invasion Assay Kits are powered by VitroGel® hydrogels (versatile, xeno-free, bio-functional hydrogels that closely mimic the physiological extracellular matrix) coupled with our premium quality VitroPrime™ Cell Culture Inserts, allowing more accurate and consistent invasion and migration studies than animal-based ECM.
VitroPrime™ Spread Attach Plates
Unique surface treated for superior hydrogel spreading, adherence,
and uniform surface to eliminate the edge effect, solve the floating issue, and uneven cell attachment to help promote rapid cell growth and improve cell yields.
VitroPrime™ Cell Culture Inserts
It closely mimics the in vivo environment, promoting enhanced attachment, growth, and differentiation of a wide range of cell types.
Publications
- Simões, B. M., Pedley, R., McCloskey, C. W., Roberts, M., Reed, A. D., Twigger, A.-J., Pirashaanthy Tharmapalan, Caruso, A., Cabral, S., Wilby, A. J., Harrison, H., Zhou, Y., Greenhalgh, A., Alghamdi, S. A., Forestiero, M., Lopez-Muñoz, J., Roche, J., Ren Jie Tuieng, Khan, M. A., & Squires, S. (2025). Anti-progestin therapy targets hallmarks of breast cancer risk. Nature. https://doi.org/10.1038/s41586-025-09684-7
- Wilby, A., Cabral, S., Zoghi, N., Howell, S., Farnie, G., & Harrison, H. (2024). A novel preclinical model of the normal human breast. Journal of Mammary Gland Biology and Neoplasia, 29(9). https://doi.org/10.1007/s10911-024-09562-4
- Li, H., Hou, M., Zhang, P., Ren, L., Guo, Y., Zou, L., Cao, J., & Bai, Z. (2024). Wedelolactone suppresses breast cancer growth and metastasis via regulating TGF-β1/Smad signaling pathway. Journal of Pharmacy and Pharmacology. https://doi.org/10.1093/jpp/rgae065
- Xu, L., Yang, L., Zhang, D., Wu, Y., Shan, J., Zhu, H., Lian, Z., He, G., Wang, C., & Wang, Q. (2024). Multi-omics analysis reveals the unique landscape of DLD in the breast cancer tumor microenvironment and its implications for immune-related prognosis. Computational and Structural Biotechnology Journal. https://doi.org/10.1016/j.csbj.2024.02.016
- Badawe, H. M., Jean Paul Harouz, Raad, P., Abu, K., Freije, A., Ghali, K., Wassim Abou-Kheir, & Khraiche, M. L. (2024). Experimental and Computational Analysis of High-Intensity Focused Ultrasound Thermal Ablation in Breast Cancer Cells: Monolayers vs. Spheroids. Cancers, 16(7), 1274–1274. https://doi.org/10.3390/cancers16071274
- Lei, Y., Tian, B.-H., Li, X.-X., Sun, M.-Y., Guo, X.-L., Wang, Y.-D., Zhou, H.-Q., Ma, R.-S., & Liang, H.-X. (2024). Surface charge accumulation of functionalized carbonized polymer dots selectively induces lysosomal membrane permeabilization of breast cancer cells. Chemical Engineering Journal, 494, 152710–152710. https://doi.org/10.1016/j.cej.2024.152710
- East, A. K., Lee, M. C., Jiang, C., Sikander, Q., & Chan, J. (2023). Biomimetic Approach to Promote Cellular Uptake and Enhance Photoacoustic Properties of Tumor-Seeking Dyes. Journal of the American Chemical Society, 145(13), 7313–7322. https://doi.org/10.1021/jacs.2c13489
- Li, H., Shi, W., Shen, T., Hui, S., Hou, M., Wei, Z., Qin, S., Bai, Z., & Cao, J. (2023). Network pharmacology-based strategy for predicting therapy targets of Ecliptae Herba on breast cancer. Medicine, 102(41), e35384–e35384. https://doi.org/10.1097/md.0000000000035384
- Marcel Janis Beha, Kim, J.-C., San Hae Im, Kim, Y., Yang, S., Ju Hee Lee, Yu Ri Nam, Lee, H., Park, H.-S., & Hyun Jung Chung. (2023). Bioorthogonal CRISPR/Cas9‐Drug Conjugate: A Combinatorial Nanomedicine Platform. Advanced Science, https://doi.org/10.1002/advs.202302253
- Worden, A., Uline, M. J., Shazly, T., Stern, M., & Potts, J. D. (2022). Self-Assembling Toroidal Cell Constructs for Tissue Engineering Applications. Microscopy and Microanalysis, 28(2), 543–552. https://doi.org/10.1017/S1431927622000253
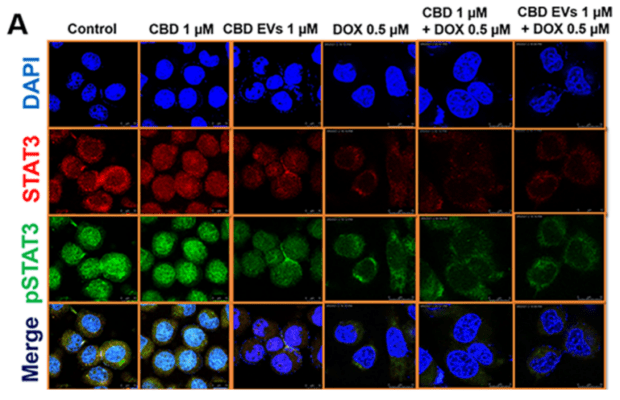
- Surapaneni, S. K., Patel, N., Sun, L., Kommineni, N., Kalvala, A. K., Gebeyehu, A., Arthur, P., Duke, L. C., Nimma, R., G Meckes, D., & Singh, M. (2022). Anticancer and chemosensitization effects of cannabidiol in 2D and 3D cultures of TNBC: involvement of GADD45α, integrin-α5, -β5, -β1, and autophagy. Drug Delivery and Translational Research. https://doi.org/10.1007/s13346-022-01137-2
- Patel, N., Kommineni, N., Surapaneni, S. K., Kalvala, A., Yaun, X., Gebeyehu, A., Arthur, P., Duke, L. C., York, S. B., Bagde, A., Meckes, D. G., & Singh, M. (2021). Cannabidiol loaded extracellular vesicles sensitize triple-negative breast cancer to doxorubicin in both in-vitro and in vivo models. International Journal of Pharmaceutics, 607, 120943. https://doi.org/10.1016/j.ijpharm.2021.120943
- Mori, H., Saeki, K., Chang, G., Wang, J., Wu, X., Hsu, P.-Y., Kanaya, N., Wang, X., Somlo, G., Nakamura, M., Bild, A., & Chen, S. (2021). Influence of Estrogen Treatment on ESR1+ and ESR1− Cells in ER+ Breast Cancer: Insights from Single-Cell Analysis of Patient-Derived Xenograft Models. Cancers, 13(24), 6375. https://doi.org/10.3390/cancers13246375
- Ogino, T., Matsunaga, N., Tanaka, T., Tanihara, T., Terajima, H., Yoshitane, H., Fukada, Y., Tsuruta, A., Koyanagi, S., & Ohdo, S. (2021). Post-transcriptional repression of circadian component CLOCK regulates cancer-stemness in murine breast cancer cells. ELife, 10, e66155. https://doi.org/10.7554/eLife.66155
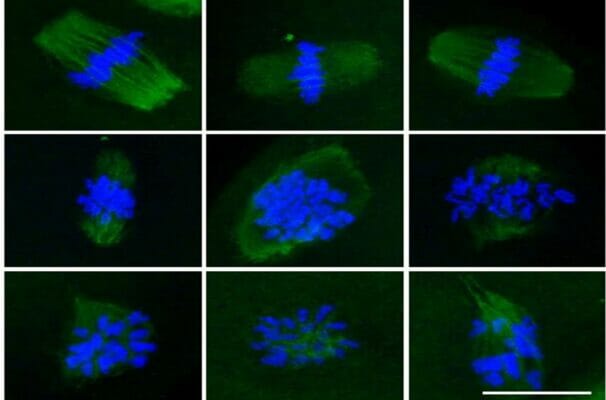
- Kim, A.-Y., Yoon, Y. N., Leem, J., Lee, J.-Y., Jung, K.-Y., Kang, M., Ahn, J., Hwang, S.-G., Oh, J. S., & Kim, J.-S. (2020). MKI-1, a Novel Small-Molecule Inhibitor of MASTL, Exerts Antitumor and Radiosensitizer Activities Through PP2A Activation in Breast Cancer. Frontiers in Oncology, 10. https://doi.org/10.3389/fonc.2020.571601
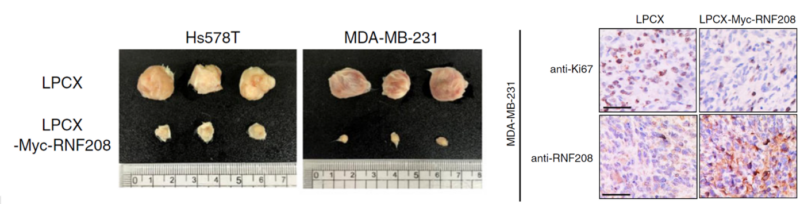
- Pang, K., Park, J., Ahn, S. G., Lee, J., Park, Y., Ooshima, A., Mizuno, S., Yamashita, S., Park, K.-S., Lee, S.-Y., Jeong, J., Ushijima, T., Yang, K.-M., & Kim, S.-J. (2019). RNF208, an estrogen-inducible E3 ligase, targets soluble Vimentin to suppress metastasis in triple-negative breast cancers. Nature Communications, 10(1), 5805. https://doi.org/10.1038/s41467-019-13852-5
- Shamloo, B., Kumar, N., Owen, R. H., Reemmer, J., Ost, J., Perkins, R. S., & Shen, H.-Y. (2019). Dysregulation of adenosine kinase isoforms in breast cancer. Oncotarget, 10(68). https://doi.org/10.18632/oncotarget.27364
- Mahauad-Fernandez, W. D., Naushad, W., Panzner, T. D., Bashir, A., Lal, G., & Okeoma, C. M. (2018). BST-2 promotes survival in circulation and pulmonary metastatic seeding of breast cancer cells. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-35710-y
- Kampert, Taylor. (2018). Combinatorial therapy for triple negative breast cancer and the effect of nanoscale surfaces. https://www.ideals.illinois.edu/handle/2142/101367(1). https://doi.org/10.1038/s41598-018-35710-y
- Mahauad-Fernandez, W. D., & Okeoma, C. M. (2018). B49, a BST-2-based peptide, inhibits adhesion and growth of breast cancer cells. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-22364-z