Research Highlights
RNF208, an Estrogen-Inducible E3 Ligase, Targets Soluble Vimentin to Suppress Metastasis in Triple-Negative Breast Cancers
Institution:
Seoul National University, Seoul, South Korea; Yonsei University Medical College, Seoul, South Korea; University of Tsukuba, Tsukuba, Japan; National Cancer Center Research Institute, Tokyo, Japan
Team:
Pang K., Park J., Ahn S.G., Lee J., Park Y., Ooshima A., Mizuno S., Yamashita S., Park K.-S., Lee S.-Y., Jeong J., Ushijima T., Yang K.-M., and Kim S.-J
Application:
Xenografting of triple-negative breast cancer cells into mice to test for tumor growth in response to the upregulation of a ubiquitin-related ligase protein.
Disease Model:
Breast Cancer
Hydrogel:
VitroGel® RGD
One of the characteristics of triple-negative breast cancer (TNBC) is the high levels of expression of the filament protein Vimentin, which exacerbates the tendency of the cells to become mesenchymal and invade other tissues. This protein controls cell adhesion and motility depending on its state of phosphorylation. Understanding the regulatory processes involved in the expression and ubiquitin-directed degradation of Vimentin would be extremely valuable in the development of diagnoses and therapies for TNBC and other aggressive cancers. In this study, an estrogen-inducible E3 ligase protein known as RNF208 was found to suppress the levels of Vimentin by directing its degradation via ubiquitination. It was hypothesized that K27-linked polyubiquitination at the Ser39 residue of phosphorylated Vimentin may ultimately result in lowered metastasis. However, this had not yet been definitively shown, and experiments were needed to demonstrate a clear mechanistic relationship between the expression levels of RNF208 and the degree of tumor growth suppression.
A joint team of researchers from Korean and Japanese cancer institutes performed a series of in vitro and in vivo experiments to investigate the expression and mode of action of the ring-finger protein RFN208. A preliminary investigation of public databases revealed a positive association between the expression of RFN208 and the estrogen receptor (ER) status, suggesting a causal relationship between the two.
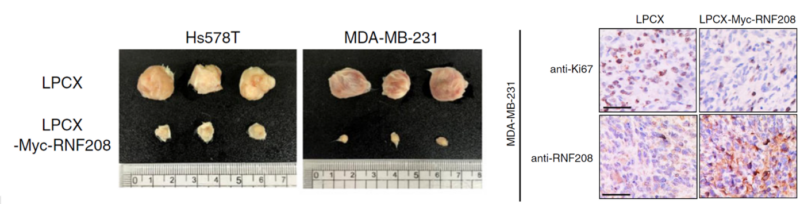
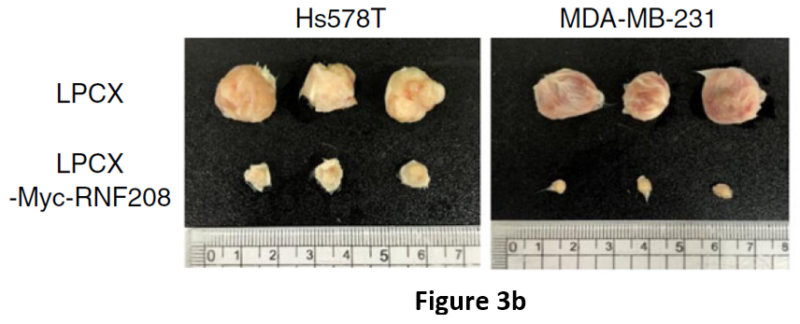 Next, the teams demonstrated that the overexpression of RNF208 reduces tumor growth and metastasis. They measured several morphological, histological, and molecular variables in immunodeficient mice injected with TNBC cells. The xeno-free injectable VitroGel® hydrogel system was used to encapsulate cells for xenografts. A comparison of Hs578T and MDA-MB-231 cells infected with a blank vector (LPCX) as a control and an RNF208-overexpressing vector was made. Importantly, expression of Vimentin was decreased in RNF208-overexpressing primary tumor tissues compared with control tissues in the xenograft mouse experiment. Tumor growth of the control and RNF208-overexpressing cells showed a marked difference (Figure 3b).
Next, the teams demonstrated that the overexpression of RNF208 reduces tumor growth and metastasis. They measured several morphological, histological, and molecular variables in immunodeficient mice injected with TNBC cells. The xeno-free injectable VitroGel® hydrogel system was used to encapsulate cells for xenografts. A comparison of Hs578T and MDA-MB-231 cells infected with a blank vector (LPCX) as a control and an RNF208-overexpressing vector was made. Importantly, expression of Vimentin was decreased in RNF208-overexpressing primary tumor tissues compared with control tissues in the xenograft mouse experiment. Tumor growth of the control and RNF208-overexpressing cells showed a marked difference (Figure 3b).
The excellent rheological features and the biological functional properties of the VitroGel system are to help cell retention and support tumor formation. For their tumor-formation assay, TNBC cells were xenografted into 6-week-old female mice. Using metastasis into the lung as a marker, the metastatic potential of the control and RNF208-enhanced cells could be contrasted (Figure 3g). VitroGel RGD in fact has finely tuned rheological properties that can permit injection hours after mixing with cells. The room temperature stable hydrogel solution can be mixed with cell suspension directly. The users can also get full control of the supplement in the hydrogel-cell mixture by adding the compounds directly to the cell suspensions. In the Pang et al. study, the authors resuspended the various TNBC cell preps in 1:3 PBS/VitroGel RGD hydrogel solution and subcutaneously injected into the mice to measure tumor growth (Figure 3g).
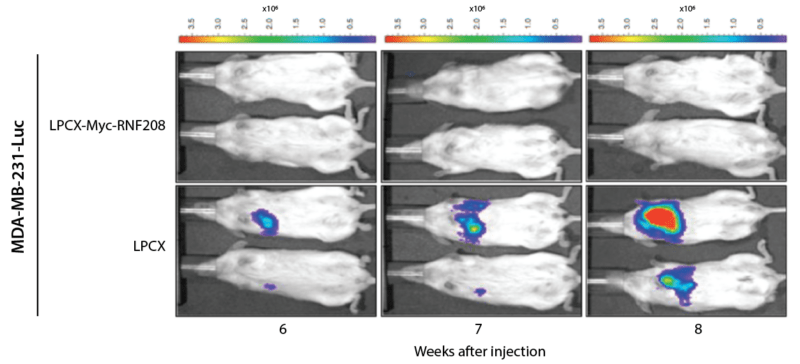
Figure 3g
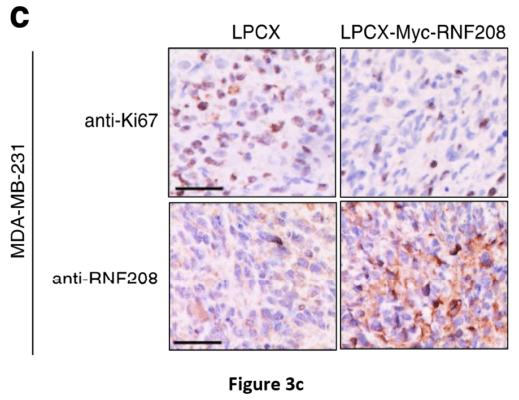
After four weeks, the lungs were stained with India ink and nodules were counted (Figure 3c). The authors observed that RNF208-overexpressing TNBC cells had a significant reduction in cell migration and invasion. The authors also utilized mass-spectrometric and RT-PCR assays to zero in on the K27-linked polyubiquitination mechanism of RNF208-directed tumor suppression. In summary, the results of this study show that RNF208 expression is closely associated with Vimentin-mediated aggressive cancer progression of breast cancer cells.