Research Highlights
VitroGel® RGD-derivatized Hydrogels Can Support Cartilage Wound Healing
TheWell Bioscience VitroGel® RGD and VitroGel® 3D speed up how fast joints can repair themselves
Institutions:
Laboratorio di Immunoreumatologia e Rigenerazione Tissutale, Bolonga, Italy
Team:
Manferdini C., Trucco D., Saleh Y., Gabusi E., Dolzani P., Lenzi E., Vannozzi, L., Ricotti, L., and Lisignoli G.
Application:
To aid the healing process of damaged joint cartilage using adipose mesenchymal stromal cells
Disease Model:
Cartilage wound healing
Hydrogel:
VitroGel® 3D
VitroGel® RGD
It has been a thought a possibility that injectable hydrogels can aid and support the healing of cartilage wounds, which on their own have a limited ability to regenerate. Hydrogels could potentially provide both a suitable environment and the biomaterial to speed along the natural process of cartridge healing. A recent Italian study focused on articular cartridge, which is the tissue that covers the ends of bones where they articulate in the joints. When this tissue is damaged as a result of trauma, aging, or other factors, they are slow to heal, in large part because they are not vascularized.
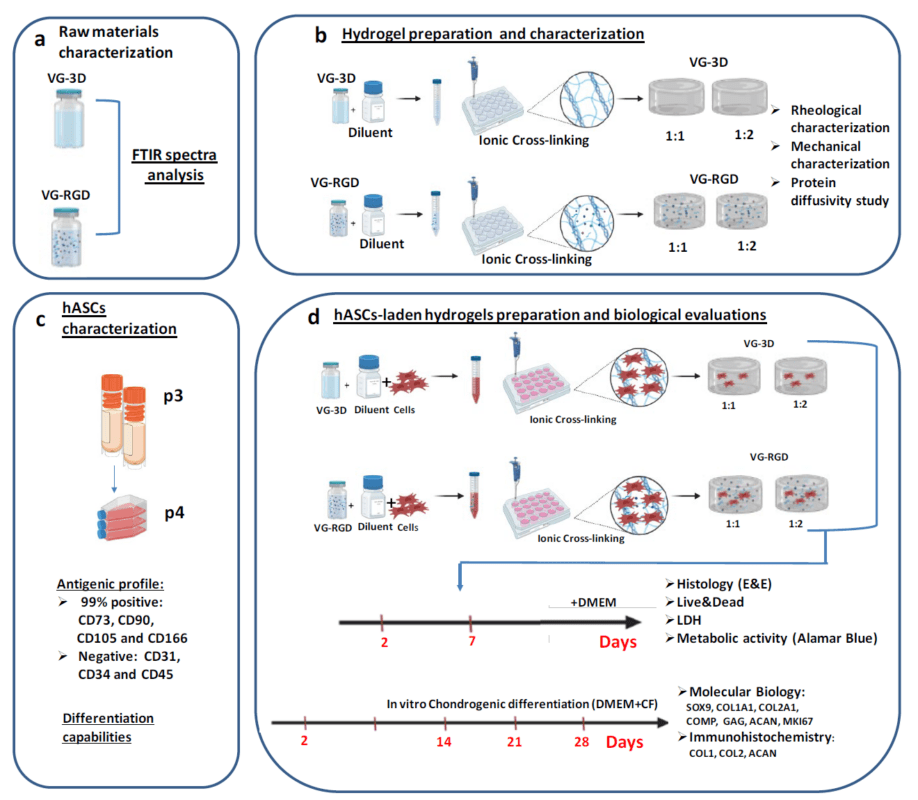
In the new paper, a team from the IRCCS Istituto Ortopedico Rizzoli in Bologna, Italy, analyzed a variety of hydrogel properties in a human adipose MSC (hASC) environment. The advances in tissue engineering inspired the authors to compare and contrast various dilutions of VitroGel® RGD and VitroGel® 3D in their injectability and ability to promote cartilage healing in mesenchymal stromal cells (MSCs). They compared the two hydrogels at two different dilution factors, and examined a suite of physical and physiological parameters. In particular, they tested a 1:1 and 1:2 dilutions of VitroGel® RGD and VitroGel® 3D made a room temperature with the Dilution Solution Type 1. These were allowed to cross-link and then incubated with hASCs for up to 28 days. At various time points, the hydrogels were assayed for rheometry (such as shearing and viscosity), cell viability, cytotoxicity, and a few indicators of gene expression changes. These values were compared to those of the hydrogels alone, without cellular co-incubation. The authors found that both hydrogels indeed could exhibit promising structures, and could support cell viability, mobility, and the expression of key proteins that are involved in would healing, such as collagen type 2. In direct comparisons, although all the hydrogel conditions examined were supportive of the conditions that support healing, a 1:2 dilution of arginine-glycine-aspartic acid-derivatized hydrogel (i.e., VitroGel® RGD) displayed the greatest ability to promote the expression of healing-related proteins.
Overall, this work highlighted the utility of hydrogel injection to augment the wound healing trajectory of human adipose MSCs. Hydrogels appear promising in the stimulation of the laden hASCs to commit to a tissue regenerative pathway. The results portend good clinical outcomes of joint damage treatments with injectable hydrogels.
Read the publication:
Manferdini C., Trucco D., Saleh Y., Gabusi E., Dolzani P., Lenzi E., Vannozzi, L., Ricotti, L., and Lisignoli G. (2022). RGD-functionalized hydrogel supports the chondrogenic commitment of adipose mesenchymal stromal cells. Gels. https://www.mdpi.com/2310-2861/8/6/382/
Related Products: