Research Highlights
3D Bioprinting of an Implantable Xeno-free Vascularized Human Skin Graft
Institutions:
Yale University School of Medicine and several other institutions
Team:
Baltazar T., Jiang B., Moncayo A., Merola J., Albanna M., Salzman W.M., and Pober, J.S.
Application:
Production of xeno-free bioink for 3D bioprinting of human skin cell grafts
Disease Model:
Human skin substitute
Hydrogel:
VitroGel® Hydrogel Matrix
In many circumstances, bioengineered skin substitutes are called for when wounds will not heal. There have been many positive clinical outcomes for cases in which human cells have been cell-cultured and then grafted back onto a patient. However, allergic responses can still sometimes be a problem, because of the xenogeneic materials used in cell culture, such as fetal bovine serum (FBS). The current study’s authors sought to overcome this issue by creating a xeno-free human skin cell culture. Moreover, the goal was to use 3D bioprinting techniques to create an artificial epidermal layer that could then be grafted back onto the host. To achieve this objective, four types of skin cells must be demonstrably cultured and bioprinted in this fashion: endothelial cells (EC), fibroblasts (FBs), pericytes (PCs), and keratinocytes (KCs). The creation of a potent xeno-free culture medium and growth conditions was also critical. In the current study, the authors turned to TheWell Bioscience’s VitroGel, as a xeno-free hydrogel in which to support the “bioink” in which the cells were grown prior to bioprinting.
For reference, bioinks are materials used to produce engineered live tissue using 3D printing. A bioink contains the cells that are to be used in a bioprinter (skin cells in this case) plus an additional medium that stabilizes the cells and helps keep them from shrinking. And a bioprinter is essentially a variant of “traditional” three-dimensional printing, except that living tissue is used as the printing material, such that a variety of living cell composites can be crafted.
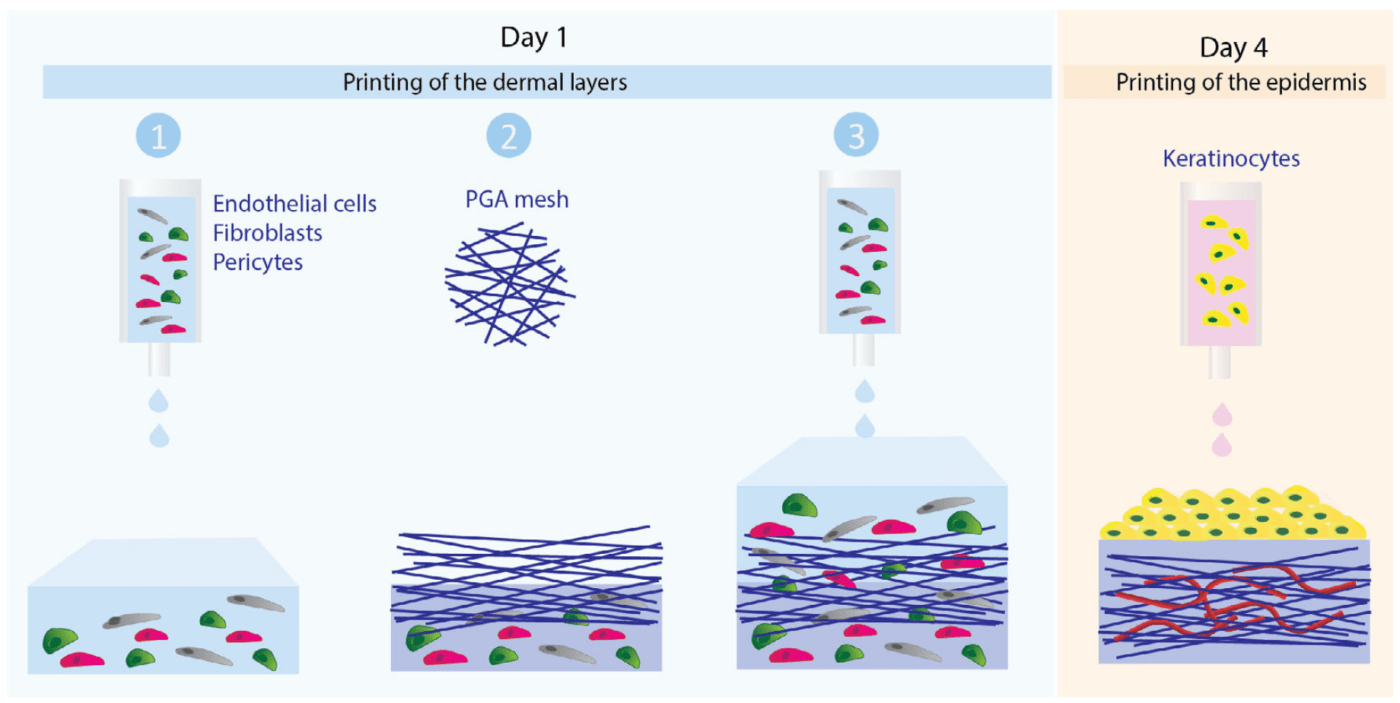
A team of immunobiologists and surgeons, coordinated by Yale University’s School of Medicine, built on their previous work in which human EC’s were shown to repopulate decellularized skin of mice. They also had success creating bioinks that could result in multi-layered and vascularized composites via 3D bioprinting. In the current study, the focus was on a xeno-free culture system that could be used to create artificial skin for people with known allergies to FBS and similar xenobiotic reagents. The researchers cultivated ECs, FBs, PCs, and KCs from human sources under xeno-free conditions. The culture medium included heparin, fibronectin, and human collagen IV. After 8-day culture and cellular immuno-characterization, the bioinks per se were put together with a mixture of FBs, ECs, and PCs, all suspended in a buffer that contained human fibronectin and collagen, plus VitroGel hydrogel for stabilization. A variety of ratios of VitroGel to collagen were tested, and the optimal ratio was chosen for bioprinting. The use of a model BioX bioprinter from CELLINK was used to layer the bioink into a mesh that could be subsequently grafted onto SCID mice. The results were very promising in that the resulting grafts supported a vascularized dermis with perfused human ECs and PCs. In sum, the process generated a differentiated multilayered human epidermis, demonstrating the utility of this approach to creating xeno-free skin grafts for use in wound healing scenarios where allergy is a concern.