Research Highlights
Choking Out Lymphoma in Dogs – Intestinal T-Cell Lymphoma
Institutions:
Rakuno Gakuen University School of Veterinary Medicine, and Osaka Metropolitan University, Japan
Team:
Yamazaki, H., Tanaka, T., Nishida, H., Hatoya, S., and Akiyoshi, H.
Disease Model:
Intestinal T-Cell Lymphoma
Hydrogel:
VitroGel® RGD
The drug evofosfamide is active under low oxygen conditions, those same conditions that tend to aid in lymphoma tumor growth. This fortuitous situation may help anticancer therapies in dogs.
Cancer cells can thrive when oxygen concentrations are low, a condition called hypoxia. One reason for this is that a particular protein, hypoxia-inducible factor 1α (HIF-1α), gets expressed at higher levels when oxygen is scarce, while it is not normally expressed very much at all under normal oxygen concentrations. The problem is that this protein can help trigger other tumor-stimulating proteins. This phenomenon has been observed in lymphoma cells, including those in non-human mammals such as dogs.
Dogs are susceptible to lymphoma and have relatively short survival times once diagnoses, even when treated with the mainline antitumor drug cocktail lomustine. The authors of this study, a Japanese team of veterinarians and oncologists, sought to understand the effects of hypoxia more thoroughly in the case of canine T-cell lymphoma and, using this knowledge, attempted to find a more potent treatment option.
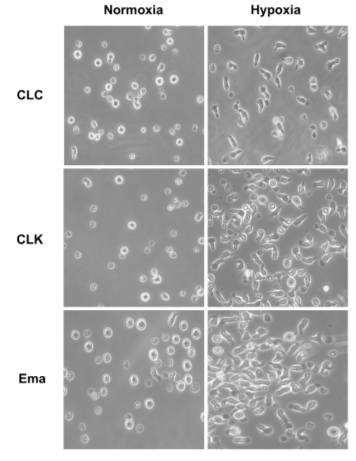
Dogs that had been diagnosed with intestinal T-cell lymphoma (ITL) and then treated with chemotherapy were chosen for initial retrospective studies; 48 individuals were broken into two groups of 24 each based on whether a histochemical assay of their lymph cells revealed high or low levels of HIF-1α protein expression. Dogs with high levels of this protein were statistically more likely to have larger tumors and develop systemic disease. By contrast, dogs with low levels of this protein were more likely to recover following chemotherapy and had a higher overall response rate. They also found that the protein tended to be localized in the nucleus under hypoxic conditions but in the cytosol under non-hypoxic (i.e., “normoxic”) conditions. This gave clues to its mechanism of action.
The researchers then moved into an experimental study of whether the drug evofosfamide (Evo), a compound that happens to release its DNA alkylating moiety (a tumor suppressor) under hypoxic conditions, could be used as a therapeutic boost in canine patients with ITL. They cultured canine ITL cell lines in TheWell Bioscience’s VitroGel® 3D-RGD to produce viable cell masses that could be studied in vitro for a response to Evo application. This 3D environment mimics in vivo cancer cell growth conditions. Notably, while hypoxic conditions spurred tumor growth in the absence of Evo, when an IC50 of this drug (ca. 50 μM) was applied for 24 hours, all three cell lines tested showed low levels of HIF-1α protein expression, which presumably could lead to better responses to chemotherapy in live patients.
While more studies need to be done in this arena, particularly on the toxicity of Evo and on the specific mechanism by which Evo exerts its effect, neither of which are fully understood, the current study shows promise for the efficacy of evofosfamide as a booster to treat ITL in dogs.