Application Notes
Quick and Efficient Organoid/Cell Recovery from Animal-Based Extracellular Matrix
Application Note
TheWell Bioscience Inc., North Brunswick, NJ

Introduction
Contemporary hydrogel matrices provide enormous advantages for two- and three-dimensional cell culturing. They provide an environment that mimics that of natural tissue, increasing growth rates and offering conditions in which native in vivo cell behavior can be reliably assayed. Choices for hydrogel matrices include those from animal-based extracellular matrices (ECMs) such as Matrigel and TheWell Biosciences xeno-free VitroGel® hydrogels. In either case, once cells have been cultured, there is often a need to recovery the cells or organoids from the hydrogel matrix for subsequent study.
Recovery of cells or organoids from hydrogels requires dedicated procedures to ensure the yield of viable products. Traditional means of recovery from animal-based ECMs pose additional challenges, however, such as the need for cold temperature, lengthy procedures, low cell viability, and restricted shelf lives of the materials used in the recovery process. Because the goal of cell or organoid recovery is often subculturing for downstream applications, it is crucial that these challenges be overcome for a wide range of users’ needs. TheWell Bioscience’s VitroGel® Organoid Recovery Solution can fulfill these needs, allowing for a room-temperature, enzyme-free recovery procedure that is performed at neutral pH and requires only 10–15 minutes to complete. This solution—and its optimized protocol—allows for harvesting cells from both 3D culture and 2D ECM coated plates.
In this comparative study, we focus on organoid harvesting from Matrigel by using a new formulation of our VitroGel® Organoid Recovery Solution, tested against our original VitroGel® Cell Recovery Solution and against another leading organoid recovery solution. We perform these comparisons utilizing different methods to assess overall robustness of the three options. Our new solution is also compatible for use in stem cell harvesting from 2D Matrigel-coated plates, which make it a powerful tool for animal-based ECM dissociation for both 3D and 2D applications.
Materials and Methods
Materials
Both organoids and cells were used to compare the efficiencies of various recovery systems and experimental conditions. Mouse intestinal organoids (MIOs) were employed for organoid recovery assays, while human iPSCs (induced pluripotent stem cells) were employed for 2D cell recovery assays. Matrigel (Corning, organoid grade: 356255) were used for initial cell/organoid culturing. VitroGel® Cell Recovery Solution (TheWell Biosciences; SKU = MS03), VitroGel® Organoid Recovery Solution (TheWell Biosciences; SKU = MS04), Solution RLR (Company SC), and Solution 3OHS (Company SA) were used as media to recover organoids or cells post culturing.
Cell Culture
MIOs were cultured in Matrigel-domed (25 μL/dome) in a well of a 24-well plate (1 mL medium per well) for three days before harvesting. For 2D culture, human iPSCs were cultured in 6-well Matrigel-coated plates for 3 days. The culture media was removed from each plate prior to organoid or cell recovery.
Recovery
For MIOs, 1mL cold (4°C) VitroGel Organoid Recovery Solution (MS04) or VitroGel Cell Recovery Solution (MS03) were added to a well of the 24-well plate to resuspend the organoid-Matrigel dome. Add another 1 mL of MS04 or MS03 to wash the plate and combine the solution to the tube. The mixture was gently pipetted, transferred to a 15 mL centrifuge tube, and chilled on ice or place in refrigerator for 2-5 minutes (MS04) or 10 min (MS03) for ECM dissociation. Solution MS03 was an original design to harvest cell from VitroGel, as was not designed for animal-based ECM use. At this point, two different agitation methods were compared. In Method 1, one that would be expected to be more suitable for expansion, the organoids were either pipetted gently up and down 5–10 times to break them into small fragments. In Method 2, one that would be expected to be more suitable for intact harvesting for downstream analysis, the tube was rocked (without using a pipette) for 2 minutes to produce intact organoids. Centrifugation at 100 x g for 3–5 min at 4 °C was performed to collect the organoids. Recovery characteristics were also compared between the two VitroGel Recovery Solutions and those from two other leading organoid recovery solutions (coded RLR and 3OHS). For these solutions, incubation time was 15 minutes, on ice, rather than the conditions listed above. The organoids harvested from all conditions were then ready to be analyzes or sub-cultured in Matrigel.
For iPSCs, after the medium was removed from 2D Matrigel-coated 6-well plates, 2 mL of PBS solution was added to each well. Then 1 mL of VitroGel Organoid Recovery Solution was added to each well. The plate was incubated for 3–5 min at 37 °C. Then 1 mL of medium was added to each well and the cell suspension was transferred to a 15 mL centrifuge tube. The plate was washed twice with additional 1 mL aliquots of medium, and then all the washes were combined into the centrifuge tube. The tube was centrifuged at 100 x g for 3–5 min at 4 °C, and the supernatant was removed, at which time the cells in the retentate were ready for subculture, analysis, or storage.
Analysis
Recovered organoids and cells were imaged at 0-, 1-, and 2-days post-recovery and during Matrigel subculturing to assess levels of intactness and overall morphology. Images were used to compare the efficacies of intact recovery of different biotic types (organoids vs. cells), different recovery solutions, and different techniques employed during the recovery, as discussed above.
Results
To begin, we assessed the effect of the type of physical agitation during organoid collection on the characteristics of the recovered organoids. Method 1, gentle pipetting, resulted in more dispersed organoids that could be easily distributed into multiple vessels, while Method 2, centrifuge tube rocking, resulted in more clumped organoids that could easily be directly harvested (Figure 1). These characteristics persisted for the entire 2-day span of analysis (Figure 1).
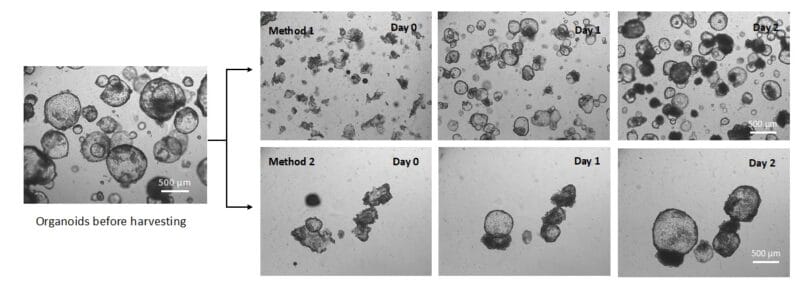
Figure 1. Organoid recovery from Matrigel using the VitroGel Organoid Recovery Solution. Two different physical recovery methods were used. Method 1 (top sequence): using a pipette to break up the organoids gently into small fragments for sub-culture/expansion. Method 2 (bottom sequence); rocking a centrifuge tube containing the recovery solution and the organoids without a pipette to recover more intact organoids.
Next, we compared the recovery between VitroGel Organoid Recovery Solution (MS04) and VitroGel Cell Recovery Solution (MS03) (Figure 2). After centrifuging to collect the organoids, the Matrigel in MS04 solution was completely dissolved, while, for the MS03 solution, there was a small amount of soft gel remaining on top of the organoids. While both solutions resulted in healthy organoid expansion and in organoids that could be subsequently harvested or sub-cultured, the newer, VitroGel Organoid Recovery Solution (MS04) demonstrated a higher recovery efficiency than the older VitroGel Cell Recovery Solution (MS03).
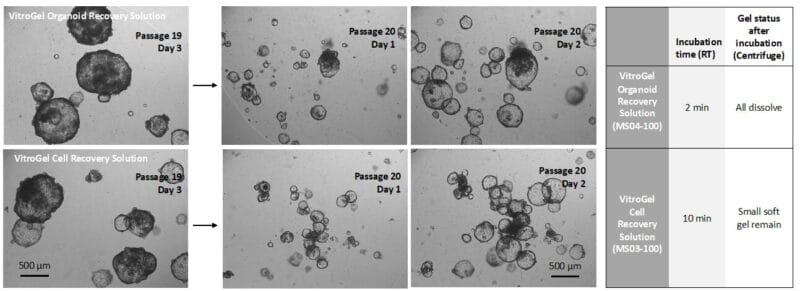
Figure 2. Comparison of VitroGel Organoid Recovery Solution (top) with the older VitroGel Cell Recovery Solution (bottom). Organoids were cultured in Matrigel for three days before harvesting. The images above show the healthy organoid expansion from both cell recovery solutions, but the newer VitroGel Organoid Recovery Solution shows a higher recovery efficiency.
To compare the VitroGel Organoid Recovery Solution to other leading recovery solutions on the market, we had to vary the incubation time and temperature, extending the former from 2 min to 15 min, and lowering the later from room temperature to an ice bath. The comparative recovery characteristics among VitroGel Organoid Recovery Solution and the RLR and 3OHS solutions in shown in Figure 3. While all of the original Matrigel was dissolved following incubation in the VitroGel Organoid Recovery Solution, most of the gel remained following incubation in RLR or 3OHS. Furthermore, resuspension via pipetting from the VitroGel Organoid Recovery Solution proved to be facile, while that from RLR or 3OHS proved difficult. As a consequence, the cell recovery rate was best from the VitroGel Organoid Recovery Solution, followed by that from RLR and then that from 3OHS. Organoids recovered from the VitroGel Organoid Recovery Solution displayed a more intact morphology after 5 days post recovery than the other two solutions (Figure 3).

Figure 3. Comparison of VitroGel Organoid Recovery Solution (top) with other organoid recovery solutions. Solution RLR was from Company SC and Solution 3OHS was from Company SA. Differences in recovery techniques are described in the main text. Unlike the cases with other two solutions, organoids recovered using MS04 solution can be more easily resuspended in cell culture medium and can be broken into small fragments more easily for subculturing.
Lastly, we analyzed the efficacy of using the new VitroGel Organoid Recovery Solution for harvesting cells from 2D Matrigel-coated plate cultures. From microscopy imaging, it was clear that human iPSCs could be readily dissociated from the plates after only 3 minutes of incubation in the recovery solution (Figure 4). Moreover, this procedure resulted in complete removal of cells from the surfaces of the microwell plate, and a robust morphology after subsequent 3-day re-culturing in Matrigel (Figure 4).

Figure 4. Use of VitroGel Organoid Recovery Solution for iPSC harvesting from 2D Matrigel-coated plates. The VitroGel Organoid Recovery Solution can be used to harvest iPSC cells from Matrigel. A) Morphology of cells detaching from the Matrigel-coated plate, 3 min after adding VitroGel Organoid Recovery Solution. B) Image of the surface of the 6-well plate after cell harvesting, showing that all cells were removed from the Matrigel-coated plate. C) Morphology of cells after re-seeding to a new Matrigel-coated plate (at day 3).
Discussion
The results shown above underscore several benefits of the VitroGel Organoid Recovery Solution for the efficient recovery of organoids and cells following culturing in an animal-based ECM (Matrigel). First, for organoids, there is a distinct time savings and ease of temperature use (i.e., room temperature or above) when the VitroGel Organoid Recovery Solution is used. Second, two distinct, but easy, methods of physical agitation can be employed with this solution to generate organoid clumping morphologies that can be tailored to different downstream applications. For example, recovery using the VitroGel Organoid Recovery Solution can easily break the organoid in small fragments for later expansion. Third, gel dissociation is easy and apparent following the use of the VitroGel Organoid Recovery Solution. If the gel does not dissociate, it is harder to pipette in order to break the organoids into small fragments. This is because the viscosity of the gel; the shearing force of pipette is not enough to break the organoid in a high viscosity mixture. However, the VitroGel Organoid Recovery Solution obviates all of these issues, and it is far more able to do so than two leading comparator recovery solutions.
We have also demonstrated the versatility of the VitroGel Organoid Recovery Solution in that it can also be utilized to achieve efficient and easy isolation of human cells following culturing on Matrigel coating plate. Thus, 2D stem cell harvesting can be achieved with gentle detachment without the use of mechanical force, granting users high cell yield and high cell viability.
In conclusion, the analyses and comparisons we perform here document that TheWell Bioscience’s VitroGel® Organoid Recovery Solution (MS04) is the superior solution for quick and efficient organoid/cell recovery from animal-based ECM.
References
- Risbridger, P., Lawrence, M. G., & Taylor, R. A. (2020). PDX: Moving Beyond Drug Screening to Versatile Models for Research Discovery. Journal of the Endocrine Society 4(11). https://doi.org/10.1210/jendso/bvaa132