Conferences, News
AACR 2023 – Annual Conference

AACR 2023 – Booth #444
We are excited to attend again this year’s AACR 2023 (American Association of Cancer Research) in Orlando, FL, hosted at the Orlando Convention Center. Please stop by booth #444 to speak with our team members about how our VitroGel® family of animal-free hydrogels can replace animal-derived ECM for your 3D cell culture research.
HYDROGEL FEATURES
- Animal-free (Xeno-free)
- Room temperature stable
- Reproducible assays
- Easy Cell harvesting
- Easy-to-use
- No lot-to-lot variability
- High-thorughput / automatable capable
APPLICATIONS
- Organoids
- Cancer Spheroids
- Stem Cells
- MSC
- Xenograft
- Invasion Assay
- Angiogenesis Assay
- Co-culture
- Bioinks

SOME of the many 3D cell applications we will be presenting:
ORGANOIDS
- VitroGel® Organoid Recovery Solution Our newly released non-enzymatic cell harvesting soltuion to recover intact organoids/cells from an animal-based ECM or our VitroGel hydrogels.
- Application Note
“Xeno-free Organoid Generation Workflow for Stem Cell Spheroids Using VitroGel STEM and VitroGel ORGANOID Hydrogel System”
XENOGRAFT
Stop by to discuss how our xeno-free VitroGel can replace animal-based ECM for PDX or CDX models.
- Application Note with HD Bioscience / a Wuxi Apptech company
“Cell Line-Derived Xenograft Using Xeno-Free VitroGel System in Comparison to Animal-based Extracellular Matrix” - Application Note with Hera Biolabs
“Comparing the Xeno-Free VitroGel to Animal-Based Matrigel for Xenograft Studies” - See poster sessions below
HIGH-THROUGHPUT SOLUTION FOR 3D CANCER STUDY
- Application Note with Molecular Devices
“Automated Dispensing, Monitoring and Assay Development of Hydrogel-based 3D Cellutlar Model” - Application Note (Protein Fluidics)
“Automated Biomarker Assays for 3D Cell Models on the Pu·MA System with Xeno-free VitroGel Hydrogel” - Application Note (Aquarray)
“Generation of Tumor Cell Spheroids in Miniaturized Nanoliter Droplets Using Xeno-free VitroGel Hydrogel Matrix” - See poster sessions below
POSTER Sessions
Click to expand to view more details on the poster session presented by collaborators/users of VitroGel.
https://www.abstractsonline.com/pp8/#!/10828/presentation/2344
Date: Sun, Apr 16, 2023
Time: 1:30 p.m.–5:00 p.m.
Session: PO.TB05.02 – Novel Models of Human Cancer
Poster Info: Section 2
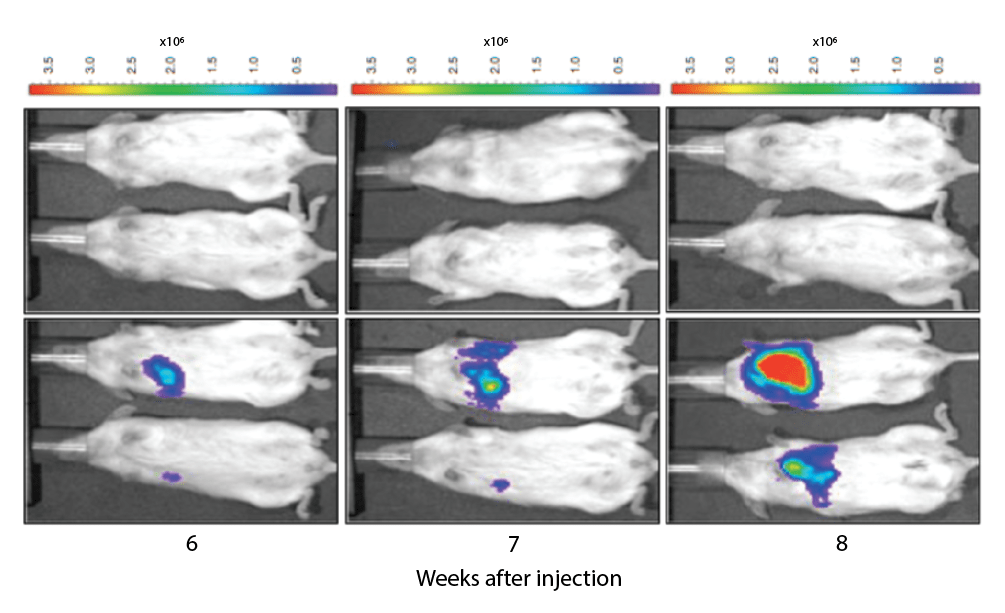
Poster: 42 / 10 – In vivo subcutaneous and orthotopic cancer xenograft modeling in the SRG immunodeficient rat
Abstract
Human cancer xenografts are a vital tool for understanding tumor biology, growth kinetics, and therapeutic efficacy using animal models. Historically, immunodeficient mice have been the standard rodent species for cancer xenograft modeling. However, an immunodeficient rat that supports a wide variety of human cancer cell types would provide a larger rodent strain for easier surgical manipulation, serial blood sampling, and provide a single model in which efficacy, pharmacokinetics, and toxicology can be performed. We have created a Sprague Dawley Rag2 -/-, Il2rg -/- rat (SRGTM OncoRat®) that provides a highly supportive environment for growing tumors of human origin. The SRG rat lacks B, T, and NK cells and readily supports the growth of multiple human cancer cell lines. The SRG rat is more immunodeficient than the Nude rat, suggesting it may be permissive to a wider variety of human cancer types. Here we demonstrate the utility of the SRG rat for both subcutaneous and orthotopic xenograft modeling. The SRG rat supports the growth of both lung and liver orthotopic cancers. In addition, the SRG rat supports the growth of orthoptic human glioblastoma multiforme in the brain. We use in vivo imaging to visualize tumor establishment and growth in subcutaneous, orthotopic, and metastatic models. Furthermore, our data show the ability of the SRG rat to support the growth of multiple different human cancer cell types subcutaneously in two different matrices, Matrigel® and VitroGel®. These data confirm that the SRG rat is an excellent host for studying human cancer. Our data demonstrate that the SRG rat has a high utility for studies using in vivo imaging, orthotopic tumor implantation, and standard subcutaneous tumor modeling. As the most immunodeficient rat commercially available, the SRG rat supports the growth of multiple human cancer types in a larger rodent strain relative to commercially available mouse models.
https://www.abstractsonline.com/pp8/#!/10828/presentation/10016
Date: Mon, Apr 17, 2023
Time: 9:00 a.m. – 12:30 a.m.
Session: LBPO – Late-Breaking Research: Prevention, Early Detection, and Interception
Poster Info: Section 36
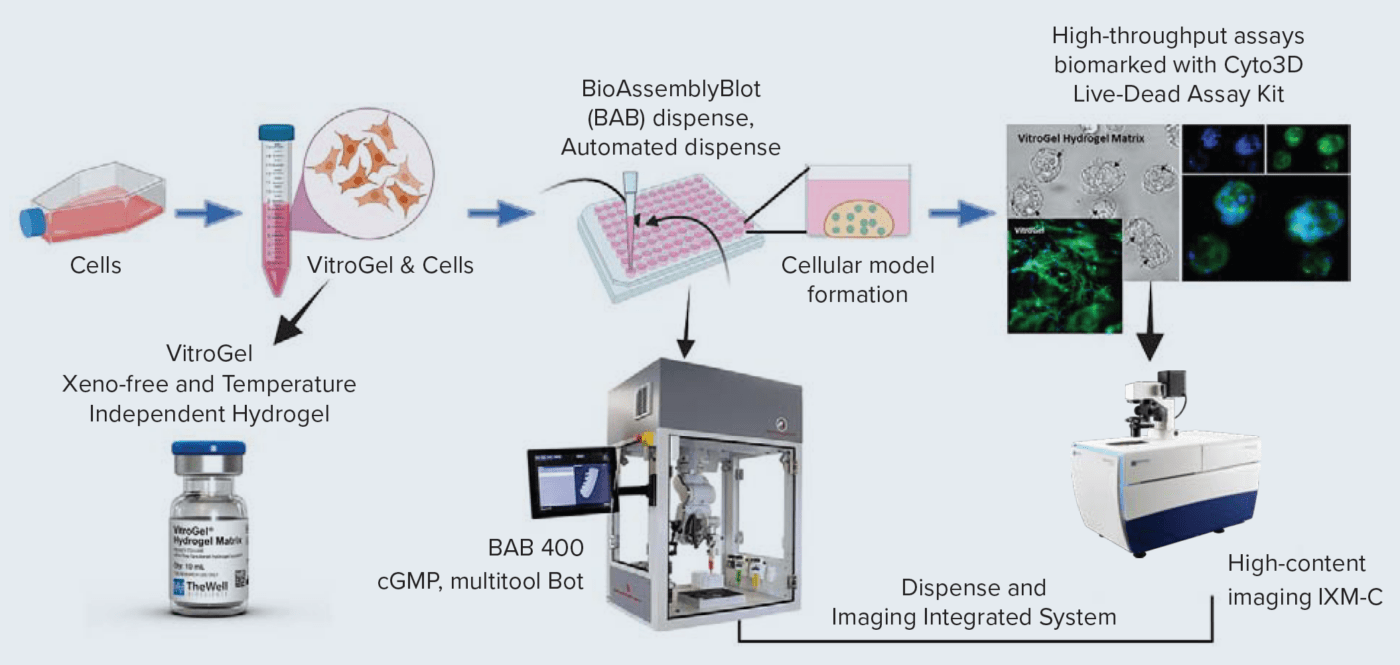
Poster: LB113 / 7 – Bio-printed 3D cell models and high-content imaging for testing anti-cancer compounds
The discovery and screening of anti-cancer drugs is a vast field that is constantly evolving. More advanced cell-based models are needed to have an efficient way of studying cellular and subcellular effects of compound treatments, especially in early drug discovery. 3D cellular models that more accurately represent various microenvironments are very important for accurate drug screening and disease modeling. However, the complexity of those models is a limiting factor for the wider adoption of 3D models for research and screening. To address this, we have developed an automated workflow that includes a multitool 3D printing/automation robot enabling printing cells in a hydrogel-based matrix and high-content imaging for the characterization of phenotypic effects of compounds. We demonstrate examples of several bio-printed 3D cell models that can be used for testing anti-cancer drugs. For the development of 3D cellular models, we used VitroGel / VitroInk, xeno-free (animal origin-free) bio-functional hydrogel matrices. Hydrogel matrices with cells were used for printing 3D cellular patterns in 96 well plates, or dispensing cells in hydrogel “domes”. We have utilized a variety of widely used cancer lines HCT-116, HELA, HepG2, and MCF-7, as well as patient-derived triple-negative breast cancer cell line 4IC. Cells mixed with hydrogels/inks were dispensed/printed into a 96-well format using the multi-tool robotic platform, BioAssemblyBot. The platform enabled efficient dispensing/printing of cells into domes, lines, or other patterns. This assay was used for compound testing and evaluation of the anti-cancer effects of various drugs. The integrated system included a high-content confocal imager (IXM-C) and enabled automated seeding, bioprinting, liquid handling, plate transferring, as well as high-content imaging. The 3D bioprinted cells were monitored daily using imaging in transmitted light. In 2-3 days, cells formed spheroids in the matrix. Those were treated with a panel of anti-cancer drugs including doxorubicin, cytarabine, taxol, mitomycin, romidepsin, cisplatin, trametinib, and other compounds with various modes of action. Cells were treated with compounds for 72h, then stained and imaged for the endpoint measurements. 3D models were stained with viability dyes including Calcein AM, EthD, and Hoechst stain to evaluate numbers of live, dead, and total cells, then imaged using the IXM-C confocal imaging system. Image analysis allowed the characterization of cytostatic and cytotoxic effects of various compounds measuring the impact of compounds on cell proliferation, number of spheroids, spheroid size, cell death, and apoptosis. Different measurements, including spheroid size, and numbers of live and dead cells were used to determine EC50s for different compounds and cell types. The results showed the feasibility of 3D cellular models bioprinted with ECM matrices for anti-cancer drug screening workflows. An increase in throughput and ease of operation was achieved through automation. Also, imaging and data analysis methods provided valuable information about complex compound effects in 3D printed and cell-tissue-engineered cancer models.
https://www.abstractsonline.com/pp8/#!/10828/presentation/2314
Date: Tues, Apr 18, 2023
Time: 1:30 p.m. – 5:30 p.m.
Session: PO.TB05.01 – 3D and Tissue Recombinant Models
Poster Info: Section 2
Poster: 4559 / 10 – Evaluation of T cell cytotoxicity, PD-L1 expression and phenotypic features in novel automated 3D microfluidic breast cancer co-culture platform
Abstract
Immunotherapy is now recognized as a powerful therapeutic approach to treat and cure cancer. However, the objective response rates for most solid tumors are low (below 30%). Accumulating evidence has shown that chemotherapy can increase the efficacy of immune checkpoint blockade and improve cancer outcomes. We have investigated if chemotherapy can modulate anti-PDL1 enhanced T cell cytotoxicity in breast cancer using novel in vitro 3D co-culture assay system. 3D co-culture of cancer and immune cells is a powerful platform for disease modeling and therapies testing because it can mimic the tumor micro-environment and complex cellular interactions. In this study we evaluated the immunomodulatory effect of chemotherapy on spheroid-T cells interactions in response to PD-L1 inhibition in triple-negative breast cancer. MCF7 and MDA231 cell lines with differential PD-L1 status were formed into spheroids and used as a tumor model. T cells were activated from PBMCs using ImmunoCult CD3/CD28 T cell Activator. Co-culture assays were performed over 72 hr in a Pu·MA System. The Pu·MA System is an automated microfluidic platform that enables phenotypic and functional assays using physiologically relevant 3D cell models. Cancer spheroids and T cells were cultured, manipulated, and measured in a single well of a microfluidic flowchip. The platform integrates 1) 3D cell model with delivery of immune cells, 2) drug delivery, 3) imaging of phenotypic features and 4) functional profiling for cytokine secretion and viability. T cells-spheroid interaction, invasion and cell viability was assessed using high-content fluorescence imaging CellVoyager CQ1 system. IL2, IFN-γ and TNF-α secretion was measured in collected supernatants using Lumit immunoassays. After viability determination, co-cultures were fixed and stained for cancer and T cell markers (E-Cadherin, F-Actin, CD3, CD8) using automated IF staining protocol and then imaged. Image analysis revealed colocalization and infiltration of the T cells into the spheroids. Disintegration of spheroid, loss of circular shape, and increased number of dead cancer cells indicated T cell mediated cancer cell death. To evaluate the immunomodulatory effect of chemotherapy on spheroid-T cells interactions in response to PD-L1 inhibition, co-cultures were exposed to PD-L1 inhibitor Atezolizumab in the presence or absence of Cisplatin/Pemetrexed. We have analyzed a shift in T cell-mediated killing activity and function in the presence or absence of chemotherapeutics. The proposed co-culture platform can be further extended to a more complex patient-derived 3D models using different cell types. Our functional immune-oncology 3D platform allows to study the crosstalk between immune, cancer and other cell interactions, evaluate new drug candidates and assess individual therapeutic approaches to advance precision medicine.
Want to schedule a time with our team at AACR or can not attend? Please fill the form and our team will be happy to assist you in any 3D cell culture applications with our VitroGel hydrogels.

