VitroINK® Bioinks
Ready-to-use bioinks for 3D bioprinting
VitroINK® Bioinks
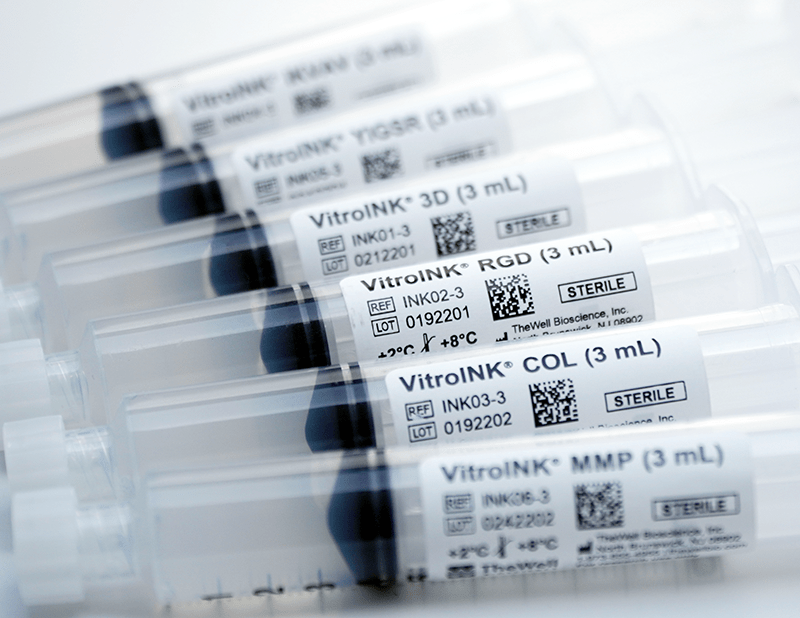
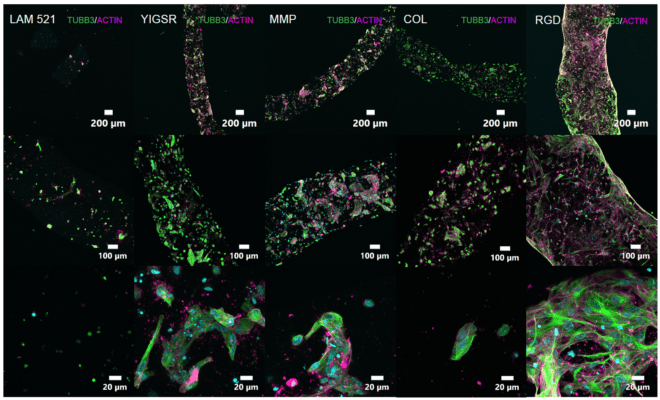
VitroINK® are ready-to-use, xeno-free (animal origin-free) bioink systems either unmodified or modified with either RGD, Collagen-memetic, IKVAV, or YIGSR peptides or with Matrix Metalloproteinases (MMP) for biodegradable bioink.
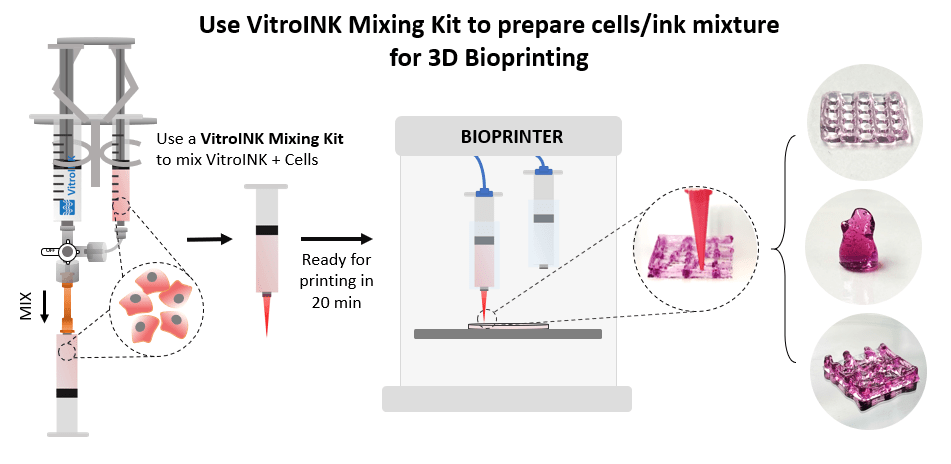
VitroINK® is a family of the ready-to-use, xeno-free tunable bioink system that requires no UV, no temperature/pH curing, or chemical cross-linking. The system is ready-to-use at room temperature, neutral in pH, transparent, and has excellent visibility after printing and cell culture. Due to the unique shear-thinning and rapid recovery mechanical property, VitroINK® can maintain the printed structure without UV or other special curing methods. Adding cell culture medium after printing can further stabilize the printed structure and support cell growth. Cells can be pre-mix with VitroINK® using our VitroINK® Mixing Kit for mixing ratios at 3:1 or 10:1. Different versions of VitroINK® may incorporate multiple biological functional ligands to promote cell attachment, cell-matrix interactions, cell proliferation, motility/migration and differentiation for many different applications.
- Need a customized bioink? Contact us for a quote.
- Need rheology data of VitroINK® with your medium and mixture for publication? Check our data support service.
Specifications
- Xeno-free tunable bioinks
- Ready-to-use at room temperature
- No UV, temperature/pH curing or chemical cross-linking required
- Neutral pH
- Transparent. Excellent visibility after printing and cell culture
- Pre-mix with cells by using our VitroINK® Mixing Kit
- Ships room temperature. Store at 2-8°C
- Sizes: 3 mL
PROTOCOLS/HANDBOOKS/RESOURCES
Product Documentation
![]() Material Safety Data Sheet (MSDS)
Material Safety Data Sheet (MSDS)
Video Protocols & Demonstrations | Application Notes | Research Highlights | Webinars
Data and References
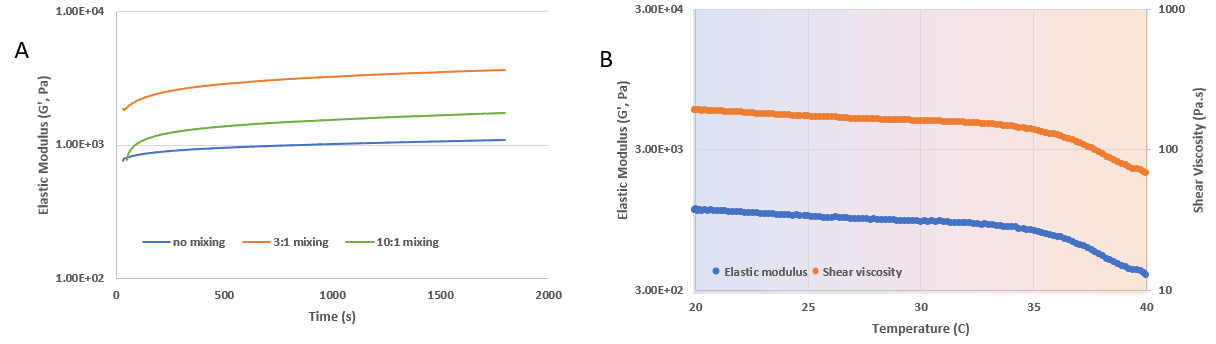
Figure 1. Rheological properties of VitroINK® RGD under the time sweep and temperature sweep tests. A) The time sweep test of VitroINK® RGD without or with 3:1 and 10:1 (v/v) mixing with DMEM medium. The elastic modulus was tested at room temperature and showed a feasible range for extrusion printing before incubation with cell culture medium. B) the elastic modulus and viscosity properties of VitroINK® RGD under the temperature sweep testing (20 to 40°C). The elastic modulus and viscosity are relatively stable at 20 to 35°C. When temperatures higher than 36, the elastic modulus and viscosity decrease. The data indicate the VitroINK has a wide range of working temperatures and can be easily used at room temperature or physiological temperature.
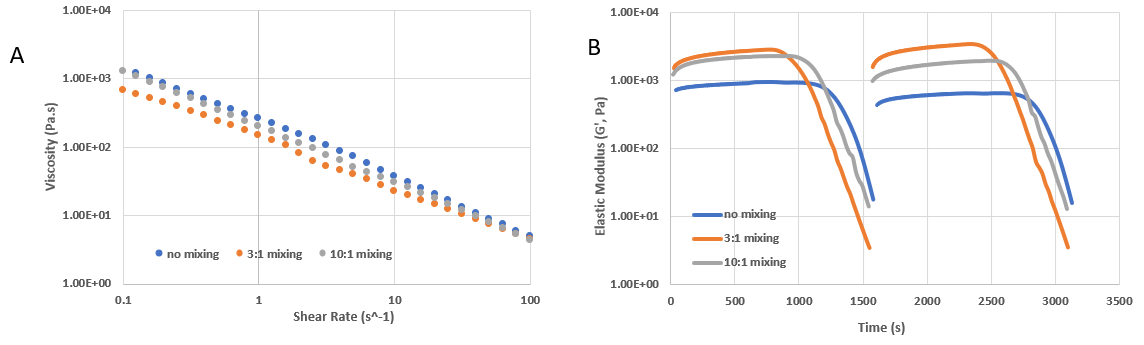
Figure 2. Shear-thinning and rapid recovery property of VitroINK® RGD. A) The shear-thinning property of VitroINK® under the shear rate from 0.1 to 100 HZ. B) the amplitude sweep test shows the shear thinning and rapid recovery property of VitroINK® under the shear strain change from 0.1 to 500% in two circles. With this unique shear-thinning and rapid recovery mechanical property, VitroINK® can well maintain the printed structure after extrusion.
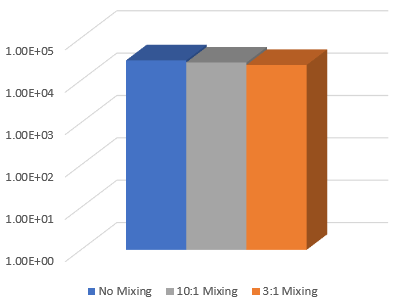
Figure 3. Gel strength of VitroINK® RGD after adding cell culture medium and overnight incubation. The VitroINK® RGD was mixed with the cell culture medium in different ratio and submerged in the medium for overnight incubating.
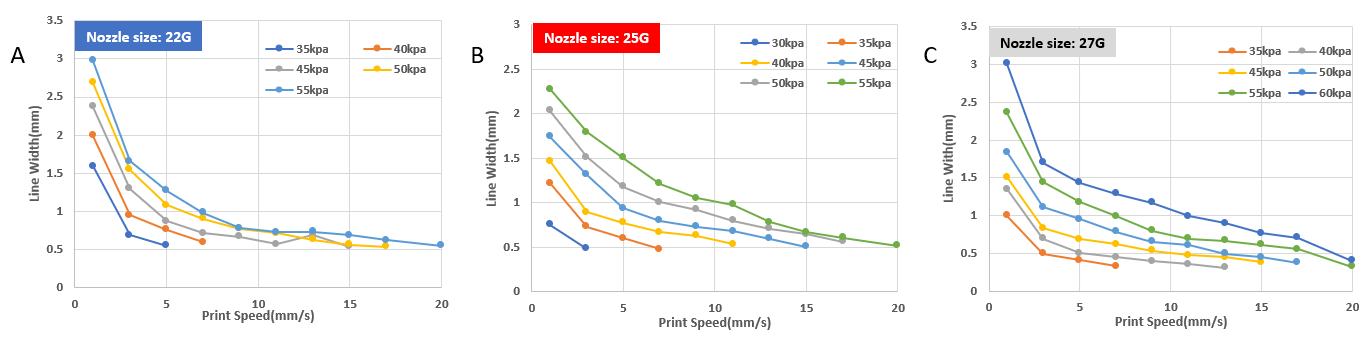
Figure 4. The printed line width of VitroINK® RGD with 22G, 25G, 27G nozzle at different pressures and print speeds. The line width decreases as the print speed increases. At lower printing pressures, lines can only be printed continuously under low print speed. (VitroINK® was printed by using Cellink INKCREDIBLE+ 3D bioprinter)
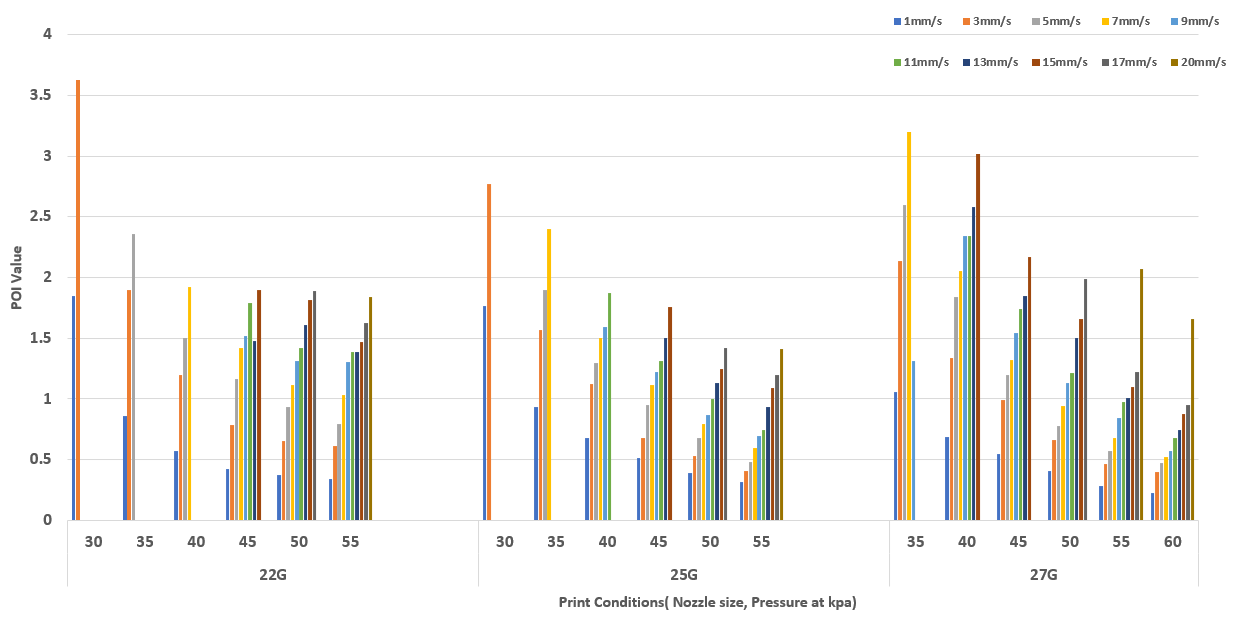
Figure 5. POI value of VitroINK® RGD with 22G, 25G, 27G nozzle at different pressures and print speeds. The printing conditions give the highest POI value of different nozzles are 30 kpa at 3 mm/s for 22G, 45 kpa at 5 mm/s for 25G and 60 kpa at 15 mm/s for 27G. Under a fixed pressure, the faster print speed gives a higher POI value.
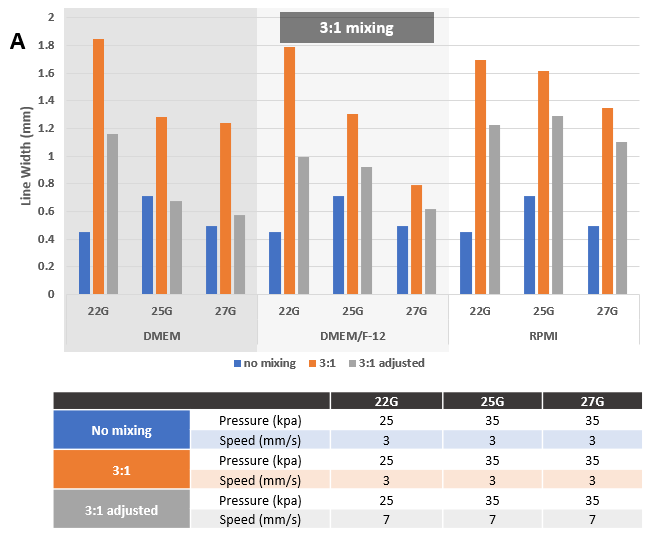
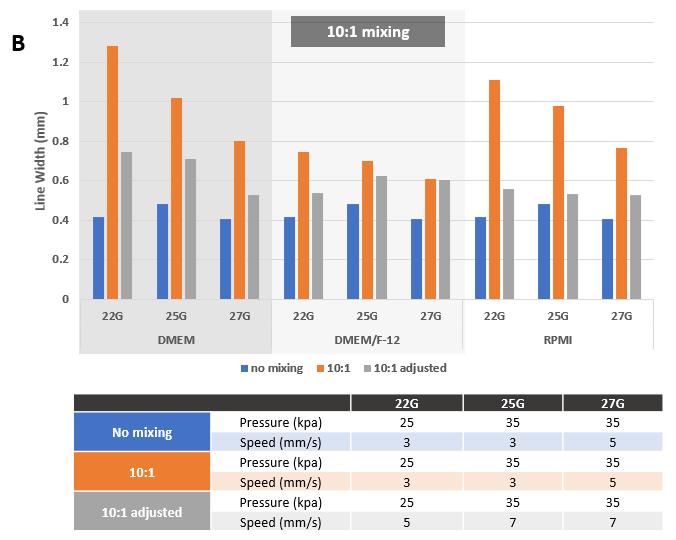
Figure 6. Adjusted printing conditions after mixing VitroINK® with Cell culture medium. Mixing at 3:1 (A) or 10:1 (B) ratios by using VitroINK® mixing kit. The line width increase after mixing. Adjust the printing speed from 3 to 7 or 5 mm/s can bring the line width down.
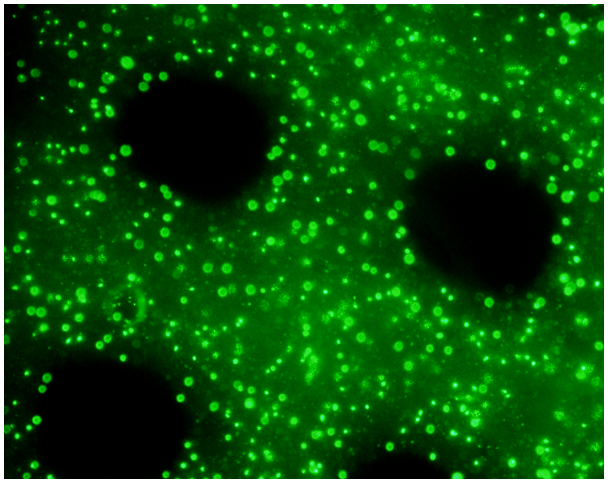
Figure 7. Patient-derived Non-Small Cell Lung Cancer (NSCLC) cells in VitroINK® RGD. Over 90% cell viability after printing.
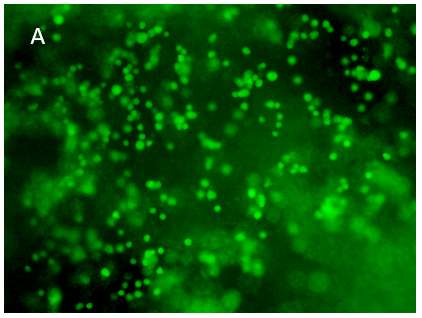
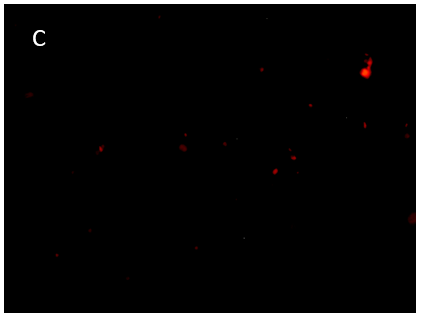

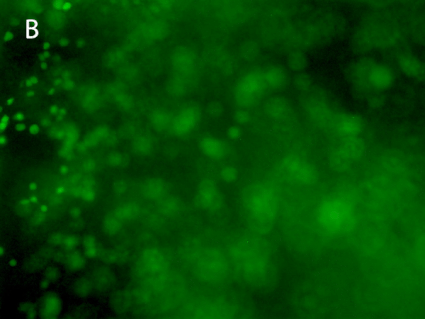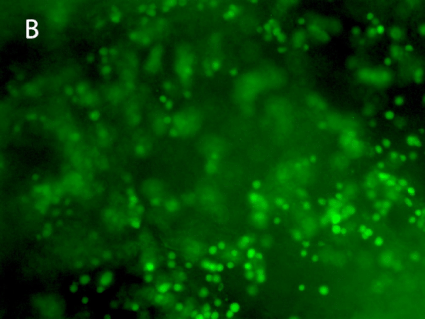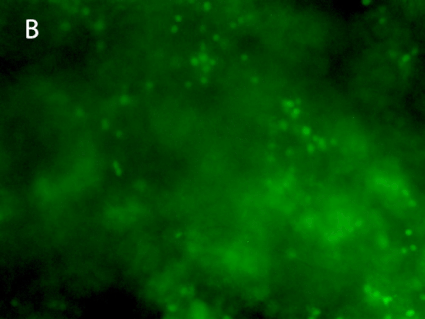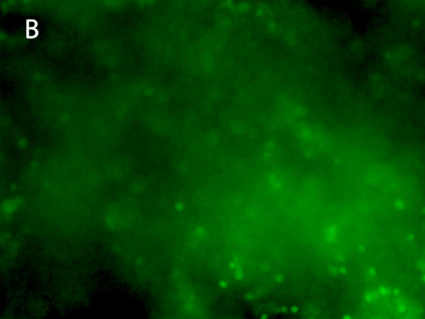

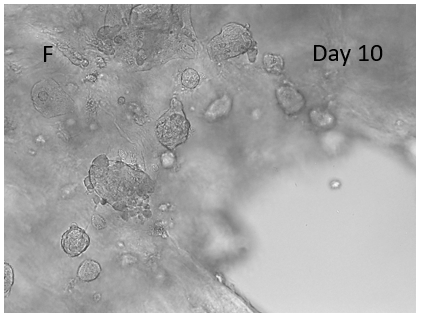
Figure 8. 3D printing of glioblastoma cell line (SNB 75) with VitroINK® RGD (3:1 bioink: cells mixing). Live/dead assay was test right after printing. The images of A) and C) show over 90% cell viability after mixing and printing. B) and D) are 3D z-stack videos of live/dead cells. E) and F) Cells grow in VitroINK® RGD on day 1 and day 10.
References/Publications
- Rosellini, E., & Cascone, M. G. (2025). Scaffolds Mimicking the Tumor Microenvironment for In Vitro Malignancy Models. Biomimetics, 10(10), 695–695. https://doi.org/10.3390/biomimetics10100695
- Pinos, R., Pauri, M., Federica Barbaglio, Di, M., Scarfò, L., Ghia, P., Conti, M., & Scielzo, C. (2025). Controlled perfusion of a vascularized microenvironment within a 3D printed bioreactor to study leukemia cells trafficking ex-vivo. Research Square (Research Square). https://doi.org/10.21203/rs.3.rs-6503832/v1
- Bilginer-Kartal, R., Çoban, B., Yildirim-Semerci, Ö., & Arslan-Yildiz, A. (2025). Recent Advances in Hydrogel-Based 3D Disease Modeling and Drug Screening Platforms. Advances in Experimental Medicine and Biology. https://doi.org/10.1007/5584_2025_851
- Robinson, M. A., Kung, S. H., Youssef, K. Y., Scheck, K. M., Bell, R. H., Sar, F., Haegert, A. M., M Mahdi Asmae, Cheng, C., Yeack, S. V., Mathur, B. T., Jiang, F., Collins, C. C., Hach, F., Willerth, S. M., & Flannigan, R. K. (2025). 3D Bioprinted Coaxial Testis Model Using Human Induced Pluripotent Stem Cells:A Step Toward Bicompartmental Cytoarchitecture and Functionalization. Advanced Healthcare Materials. https://doi.org/10.1002/adhm.202402606
- Robinson, M., & Flannigan, R. (2023). MP01-03 Xeno-Free Peptide-Functionalized Bioinks Improve Ex Vico Culture of Human Testicular Tissues. Journal of Urology, 209(Supplement 4), e1. https://doi.org/10.1097/JU.0000000000003212.03
- Thomasz Gapinski, Krzysztof Lenartowicz, Paulina Galas, Malgorzata Gonsior, Leonardo Ricotti, Lorenzo Vannozzi (2020). First tests of extrusion Process Using Arthroscopic 3D Bioprinting Handheld Tools Prototypes. Engineering of Biomaterials, 158 (220) 60. https://bibliotekanauki.pl/articles/1844936.pdf
- Gebeyehu, A., Surapaneni, S. K., Huang, J., Mondal, A., Wang, V. Z., Haruna, N. F., Bagde, A., Arthur, P., Kutlehria, S., Patel, N., Rishi, A. K., & Singh, M. (2021). Polysaccharide hydrogel based 3D printed tumor models for chemotherapeutic drug screening. Scientific Reports, 11(1), 372. https://doi.org/10.1038/s41598-020-79325-8
| Bioink Type | VitroINK® 3D (3 mL), VitroINK® RGD (3 mL), VitroINK® COL (3 mL), VitroINK® IKVAV (3 mL), VitroINK® YIGSR (3 mL), VitroINK® MMP (3 mL) |
|---|