VitroGel® Organoid Recovery Solution
Non-enzymatic cell harvesting solution to recover cells/organoids from hydrogel or an animal-based ECM within 15 minutes.
Improved formulation over VitroGel® Cell Recovery Solution.
VitroGel® Organoid Recovery Solution
(Improved Formulation over VitroGel® Cell Recovery Solution)

High cell recovery and viability
Enzyme-free and stable formulation yields high-quality cells/organoids for downstream analysis and expansion.

Long shelf-life
Stable for 24 months. No dry ice/cold shipping.

Works with cells/organoids cultured from animal-based ECM
As quick as 2-minute dissociation of animal-based ECM.
Less than 10 minutes cell recovery protocol.

Ideal for cells/organoids cultured from VitroGel® hydrogels
<15-minute cell recovery protocol.
Safe and easy-to-use.
VitroGel® Organoid Recovery Solution is a non-enzymatic cell harvesting solution for quick and efficient recovery of 3D cells or organoids cultured with either VitroGel® hydrogels or an animal-based ECM.
For cells/organoids cultured in an animal-based ECM, achieve fast 2-minute ECM dissociation safely and efficiently.
VitroGel® Organoid Recovery Solution is room temperature stable and has a neutral pH. The solution can maintain high cell viability during the recovery process. Harvested cells can be subcultured in both 3D and 2D cultures. The VitroGel® Organoid Recovery Solution can be used before or after the fixation and stained preparation of hydrogel specimens to ensure high-quality downstream data analysis.
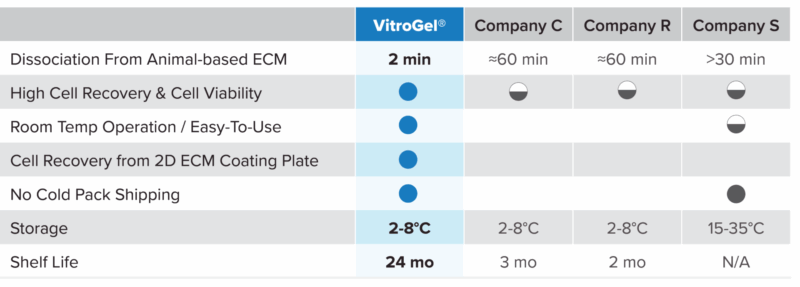
VitroGel® Organoid Recovery Solution compared to other recovery solutions
Specifications
| Sizes | 100 mL, 500 mL |
| Formulation | Enzyme-free. Free of phenol red. Sterile. |
| Use | Harvest organoids/cells cultured from animal-based ECM or from VitroGel hydrogels. Use before or after sample fixation and stained preparation for imaging or downstream data analysis |
| Processing Time | 10-15 min |
| Downstream | Recovered cells can be sub-culture in both 3D and 2D culture |
| pH | Neutral |
| Storage | 2-8 °C |
| Stability | 24 months from date of manufacture |
| Shipping | Ambient temperature |
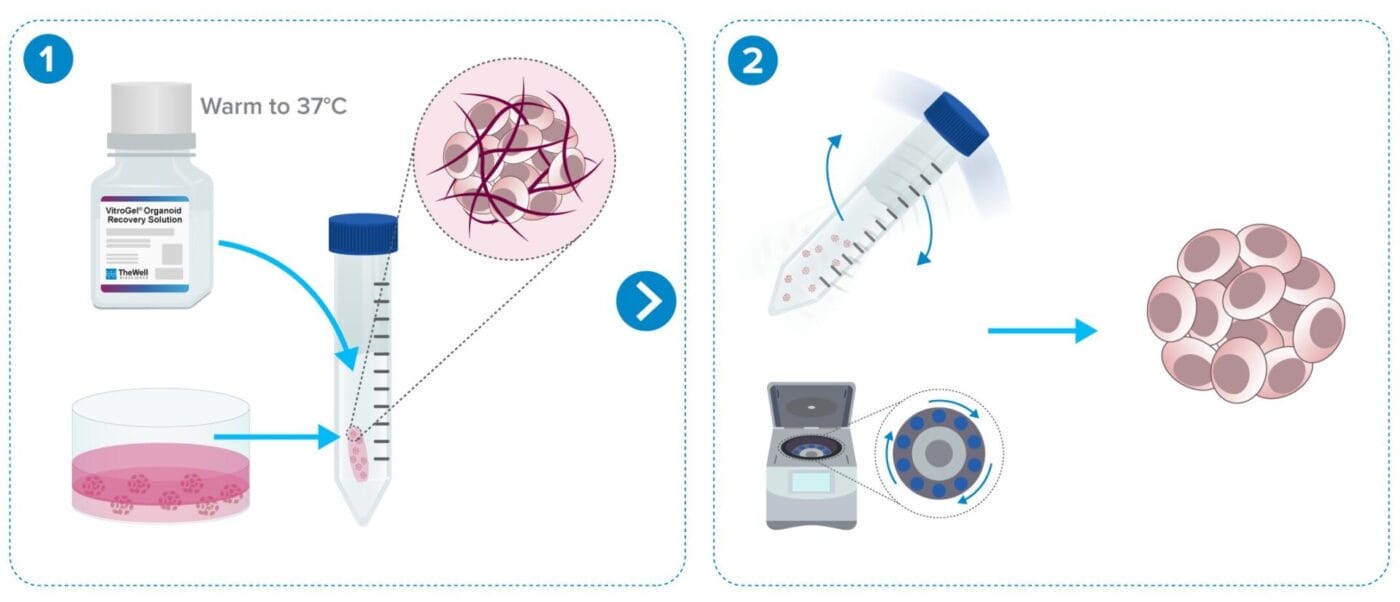
Organoid/Cell Recovery Protocol Workflows
From Animal-based ECM (e.g. Matrigel)
Less than 10 min protocol. Fast 2-minute ECM dissociation.
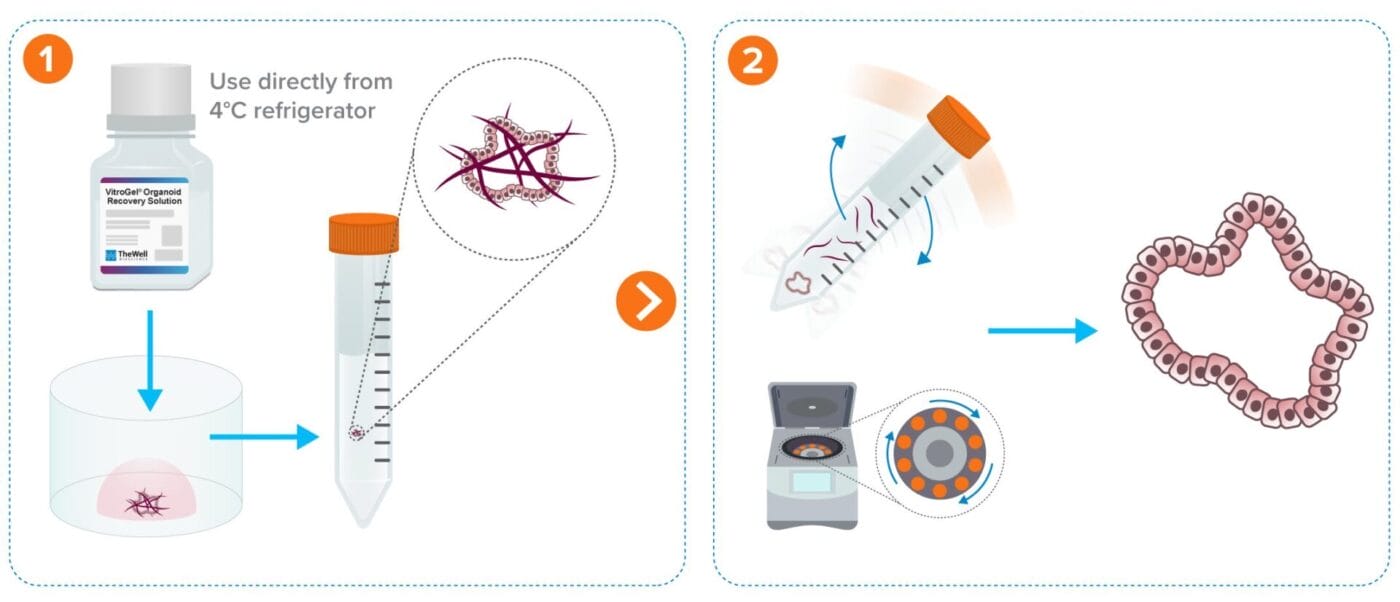

Add recovery solution to the cells and transfer to a tube.

Rock tube for 2 minutes and centrifuge.
From VitroGel® Hydrogels
Less than 15 min protocol.
Video Protocols & Demonstrations
Application Notes
Data and References
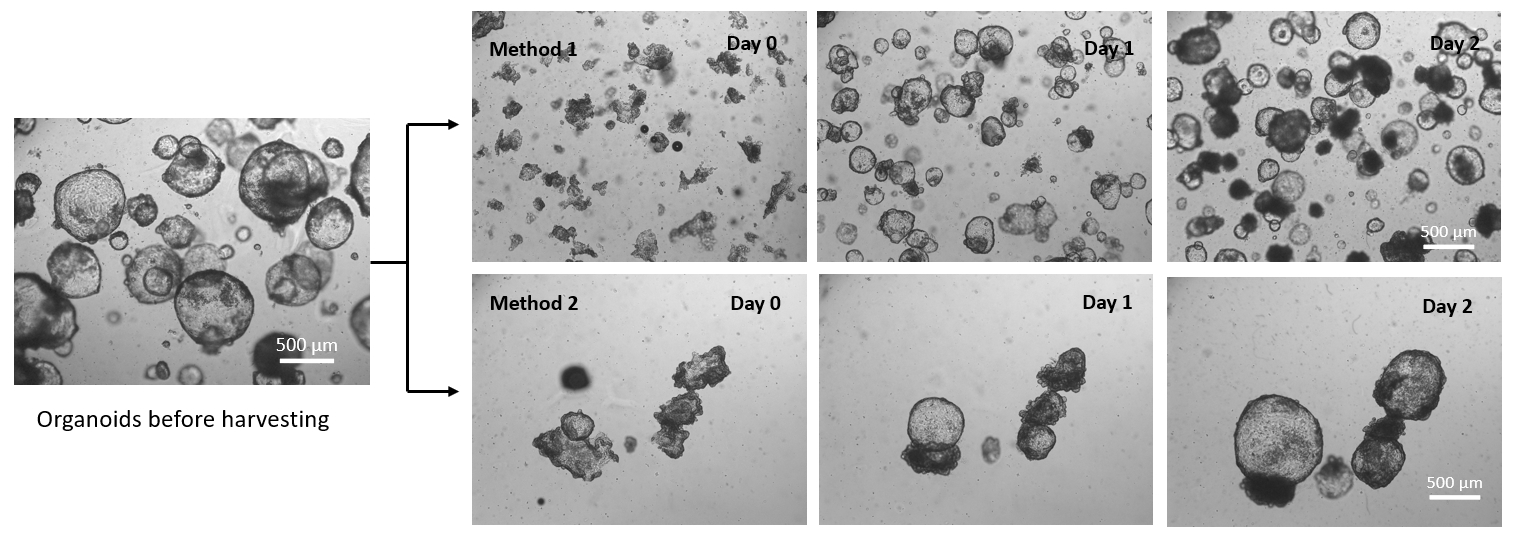
Figure 1. Organoids recovered from Matrigel using VitroGel® Organoid Recovery Solution with two methods.
Method 1: Re-suspend organoids in VitroGel® Organoid Recovery Solution by pipetting to break organoids into small fragments for sub-culture/expansion.
Method 2: Rocking the tube with organoids and VitroGel® Organoid Recovery Solution mixture without using a pipette to harvest the intact organoids.
In both, VitroGel® Organoid Recovery Solution was kept in a 4°C refrigerator to maintain a low temperature before use. The organoids/Matrigel and VitroGel® Organoid Recovery Solution mixture were incubated at room temperature for 2 min before centrifuging. Day 0 images show the morphology of organoids right after harvesting with two different methods.
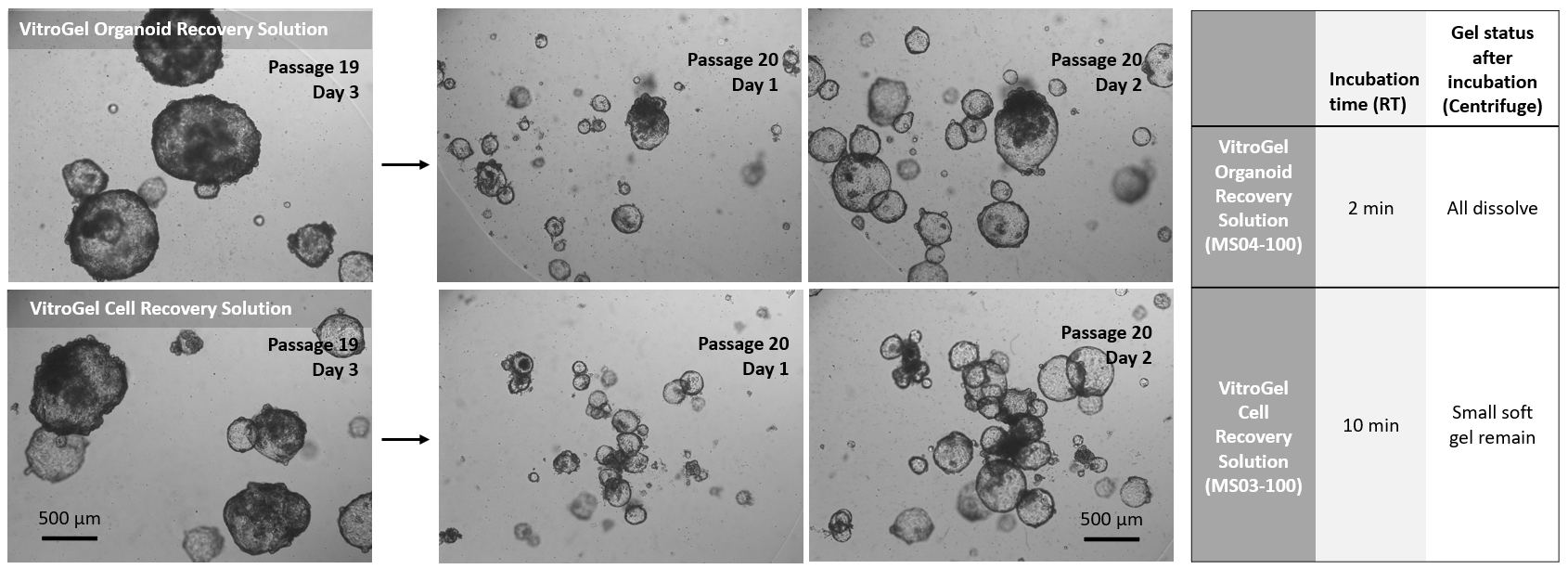
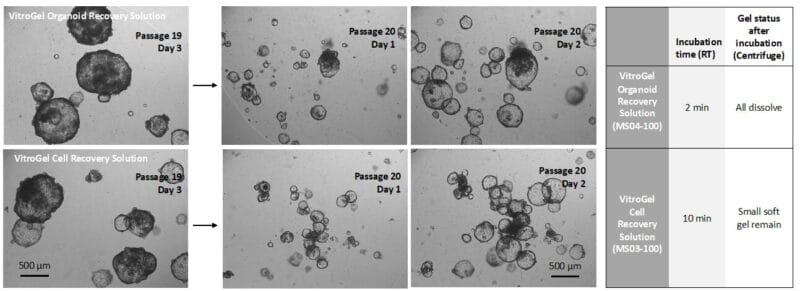
Figure 2. Comparison of VitroGel® Organoid Recovery Solution and VitroGel Cell Recovery Solution for organoids recovered from Matrigel.
Organoids (Passage 19) were cultured in Matrigel for three days before harvesting. 1 mL cold (4°C) VitroGel® Organoid Recovery Solution (MS04) or VitroGel Cell Recovery Solution (MS03) was added to a well of a 24-well plate to resuspend the organoid-Matrigel dome. The mixture was gently pipetted, transferred to a centrifuge tube, and incubated at room temperature for 2 min (MS04) and 10 min (MS03), respectively. After centrifuging to collect the organoids, the Matrigel in MS04 solution was completely dissolved. For the MS03 solution, there was a small amount of soft gel remaining on top of the organoids. The organoids harvested from both conditions were sub-cultured in Matrigel. The images above (Passage 20, Days 1&2) show the healthy organoid expansion from both cell recovery solutions. VitroGel® Organoid Recovery Solution shows a higher recovery efficiency than the VitroGel® Cell Recovery Solution in this study.
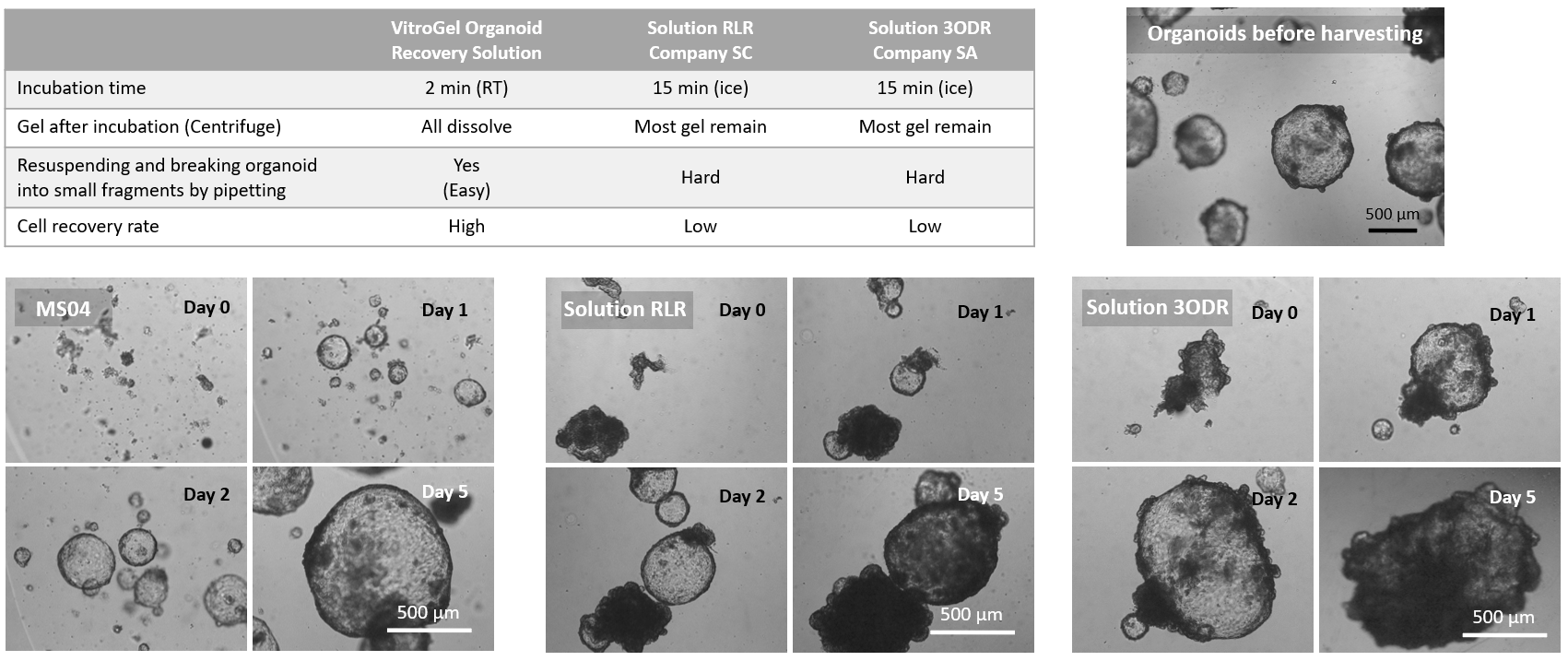
Figure 3. Comparison of VitroGel® Organoid Recovery Solution with other organoid recovery solutions.
The data above shows the comparison study of VitroGel® Organoid Recovery Solution (MS04) with other organoid recovery solutions (Solution RLR from Company SC and Solution 3ODR from Company SA). All solutions were used directly from a 4°C refrigerator. After mixing with organoid/Matrigel, the mixture with MS04 was incubated at room temperature for 2 min, while the mixture with Solution RLR or Solution 3ODR was incubated in an ice bucket for at least 15 min. After centrifuging, all Matrigel in the MS04 mixture was dissolved, but most Matrigel remained in both Solution RLR and Solution 3ODR conditions. Besides recovering intact organoids, the organoids from the MS04 solution can be easily resuspended in a cell culture medium and broken into small fragments for a subculture (MS04 images above). However, the organoid/Matrigel mixture was hard to break in Solution RLR or Solution 3ODR. VitroGel® Organoid Recovery Solution delivers a higher cell recovery rate for organoid expansion.

Figure 4. Use VitroGel® Organoid Recovery Solution for iPSC harvesting from 2D Matrigel coating plate
VitroGel® Organoid Recovery Solution can be used to harvest iPSC cells from a 2D Matrigel coating plate. The solution was warmed up to room temperature before use. A) Morphology of cells detaching from the Matrigel coating plate. (3 min after adding VitroGel® Organoid Recovery Solution), B) Image of the well plate after cell harvesting. (Shows all cells were removed from the Matrigel coating plate), C) Morphology of cells after re-seeding to a new Matrigel coating plate (Day 3).
References/Publications
- Joshi, A., Ratnapradipa, N., Hughes, J., Moore, E., Ekpenyong, A., & Shukla, S. (2025). Isoform-specific vs. pan-histone deacetylase inhibition as approaches for countering glioblastoma: an in vitro study. Frontiers in Oncology, 15. https://doi.org/10.3389/fonc.2025.1695552
- Lacombe, J., Dunn, S. E., Layac, M., Soldevila, M., Fakrudin, N. M., Duane, B., Chen, K., Barrett, M. W., Helton, J., Laiakis, E. C., Fornace, A. J., Jani, S., Sorensen, S., Funaki, S., Diaz, A., & Zenhausern, F. (2025). A human 3D culture-organ-on-chip platform for investigating the tumor microenvironment response to ionizing radiation. IScience, 29(1), 114236–114236. https://doi.org/10.1016/j.isci.2025.114236
- Admella, J., Alcàcer-Almansa, J., Julián, E., & Torrents, E. (2025). Optimized alveolar epithelial cell model for chronic Pseudomonas aeruginosa and Staphylococcus aureus coinfections. IScience, 113620. https://doi.org/10.1016/j.isci.2025.113620
- Premathilake, H. U., Mazucanti, C. H., Yao, Q., O’Connell, J. F., Nandita Vegesna, Dimitrios Tsitsipatis, Weller, C., Lam, K.-W. G., Candia, J., Fan, J., De, S., Sen, P., Egan, J. M., & Doyle, M. E. (2025). Pig Taste Cell-Derived Organoids Synthesize Insulin. Endocrinology. https://doi.org/10.1210/endocr/bqaf126
- Kim, M., & Kim, M. (2025). TRPML3‑mediated lysosomal Ca2+ release enhances drug sequestration and biogenesis, promoting osimertinib resistance in non‑small cell lung cancer. Oncology Reports, 54(3), 1–13. https://doi.org/10.3892/or.2025.8946
- Maillard, J., Pickard, L., & Banerji, U. (2025). An enzyme-free alcohol-based organoid harvesting solution. BioTechniques, 1–11. https://doi.org/10.1080/07366205.2025.2527540
- Gómez-Álvarez, M., Agustina-Hernández, M., Francés-Herrero, E., Bueno-Fernandez, C., Alonso-Frías, P., Corpas, N., Faus, A., Pellicer, A., & Cervelló, I. (2025). Generation of healthy bovine ovarian organoids: a proof-of-concept derivation technique. Journal of Ovarian Research, 18(1). https://doi.org/10.1186/s13048-025-01673-8
- Andersen, M. S., Nielsen, A. Y., Wirenfeldt, M., Petersen, J. K., Møller, M. W., Powell, C. L., Castro, A., Herrgott, G., Mathiesen, T., Poulsen, C. A., Olsen, B. B., Boldt, H. B., Pedersen, C. B., Halle, B., & Poulsen, F. R. (2025). Establishment of a patient-derived 3D in vitro meningioma model in xeno-free hydrogel for clinical applications. Acta Neuropathologica Communications, 13(1). https://doi.org/10.1186/s40478-025-02008-w
- Patel, D. P. (2025). Developing and Validating Brain Tumor Organoid Models to Evaluate Novel Therapeutics In Vitro. Scholar Commons. https://scholarcommons.sc.edu/senior_theses/741/
- De Donato, M., Babini, G., Mozzetti, S., Buttarelli, M., Ciucci, A., Arduini, G., De Rosa, M. C., Scambia, G., & Gallo, D. (2020). KLF7: a new candidate biomarker and therapeutic target for high-grade serous ovarian cancer. Journal of Experimental & Clinical Cancer Research, 39(1). https://doi.org/10.1186/s13046-020-01775-9
- Lan, T., Guo, J., Bai, X., Huang, Z., Wei, Z., Du, G., Yan, G., Weng, L., & Yi, X. (2020). RGD-modified injectable hydrogel maintains islet beta-cell survival and function. Journal of Applied Biomaterials & Functional Materials, 18, 228080002096347. https://doi.org/10.1177/2280800020963473
- Powell K. Adding depth to cell culture. Science, 356(6333), 96–98. https://doi.org/10.1126/science.356.6333.96
| Size | 100 mL, 500 mL |
|---|