Research Highlights
Going 3D to Fight Cancer
Institutions:
Rutgers
Team:
Bhatt, R., Ravi, D., Evens, A.M., and Parekkadan, B.
Application:
Cancer Research
Disease Model:
Lymphoma
Hydrogel:
VitroGel® Hydrogel Matrix
Cells propagated in a hydrogel 3D cell culture grow more slowly than they do in 2D cultures but exhibit more biological realism which is critical for cancer drug screening and therapy.
Cancer progression is a very complex process, complicated by the fact that variations from patient to patient––and even among tumors in the same patient––can prevent accurate diagnosis and treatment. Recent studies have given hope, however, to therapies that target something that may be common to all tumors: metabolic reprogramming. This is the characteristic dependency of tumors on a highly alerted metabolic environment, such as a local enrichment in particular amino acids.
The ability of oncologists and clinicians to study the metabolic behavior of a tumor depends on a reliable model system. Such a system has traditionally been either a biopsy or a 2D cell culture. In the latter case, many therapeutic predictions have been made on the response of a tumor to drug candidates. Unfortunately, because of the nature of cell growth in 2D culture, some of these predictions can be misleading. Tumor growth in a classic 2D cell culture can vastly outpace that in the body, leading to conclusions that turn out to be improper for a particular patient. Drug resistance conclusions are especially prone to this type of error. Thus, there is a concrete need to study drug resistance and growth patterns in a 3D cell culture, which can more accurately mimic an in vivo environment.
A team of oncologists from Rutgers University set out to study the tendency of diffuse large B-cell lymphoma (DLBCL) to evade therapy by developing resistance to oft-used chemotherapeutic drugs such as rituximab and obinutuzumab. They sought a 3D cell model as a comparison to the results that would be obtained in a 2D cell culture. To follow tumor growth and drug response they tracked the metabolic switching of cells to sugars, specifically glucose.
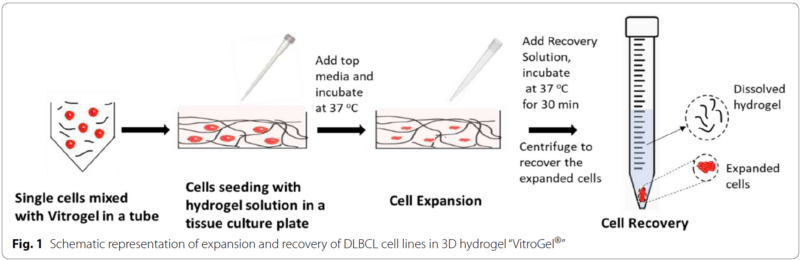
The authors chose TheWell Biosciences’ VitroGel® xeno-free hydrogel matrix as their 3D growth medium. They grew three types of lymphoma cell lines in both traditional 2D cell culture and in VitroGel®, the latter with a gel-to-cell ratio of 2:1 for five days. The parental SUDHL-10 lymphoma cell line was compared to two drug-resistant lines, SUDHL-10RR (rituximab drug-resistant) and SUDHL-10OR (obinutuzumab drug-resistant). Along the time course, cells were assayed for their ability to proliferate, what stage of the cell cycle they were in, their apoptosis status, their sensitivity to drugs, and their metabolic profile. The most noticeable result was that cells incubated in 3D culture demonstrated slower growth and a more constrained proliferation. However, these VitroGel®-grown cells retained a differential sensitivity to a drug screen, and their sugar-based metabolic profile was more akin to that of clinical cell specimens. As a result, the authors concluded that culturing cells in a 3D medium was statistically more reflective of tumor growth that would occur in the body, and thus was a better model for therapeutic tests and decisions. Despite the slower growth that cells exhibit in a 3D medium, the metabolic interactions with the environment that this condition promotes are more biologically realistic and more reflective of “real-world” physiology.