Research Highlights
Centrosome Positioning in Migrating Dictyostelium Cells
Institution:
Ludwig Maximillian University, Munich, Germany
Team:
Ishikawa-Ankerhold H., Kroll J., van den Heuvel., Renkawitz J., and Müller-Taubenberger A.
Application:
New insight into how cells move through the environment in an amoeba-like fashion as sometimes occurs during wound healing.
Disease Model:
Wound healing
Hydrogel:
VitroGel® Hydrogel Matrix
When cells move from one place to another, be it in the body or in the ecological environment, they can do so in a few different manners. For example, mesenchymal cells such as those involved in tumor growth, have a distinct front and back, and migrate in a slow fashion that involves iterative sticking to local substrates (adhesion) and continual internal remodeling. On the other hand, other types of cells such as leukocytes and dendritic cells, migrate in a manner that is characteristic of slime molds: they move faster and emulate amoeba as they navigate their locales. The centrosome is an organelle that is integrally involved in cell division and progression around the cell cycles. These structures organize the microtubules that provide cellular rigidity. Recently it has become clear that the centrosomes and their associated microtubules also influence cell translocation in the natural environment, something that has been known for decades with respect to cancer cell migration. A complex interplay between the internal cellular environment, and the external environment determines cell movement, and this process is mediated through cAMP-based cell signaling actions.
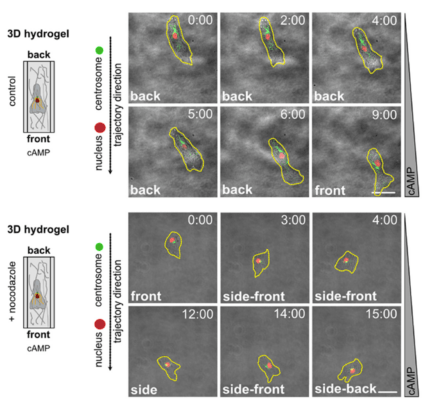
In a new study out of LMU, the authors used Dictyostelium discoideum cells to model human leukocyte cell movements in both 2D and 3D environments. They examined subcellular events, such as the position of the centrosome relative to the nucleus, while the cells moved. To emulate the 3D milieu, TheWell Bioscience’s VitroGel® Hydrogel Matrix was employed, as well as one provided by a matrix comprised of rat collagen type I. The goal was to determine whether centrosome positionings are similar in slime molds and human leukocytes, an understanding of which could help design clinical treatments for white cell disorders. The authors examined the relative locations of the centriole and the nucleus as the slime mold Dictyostelium moved through a gradient that was created by a concentration of the chemoattractant cAMP (or folic acid). This is direction migration, for which parallels can be observed across disparate cell types. In this type of movement, the cell continually “probes” its environment for spaces in which it can fit. The authors created a 3D matrix with the used of VitroGel® Hydrogel and compared the movement process through this matrix to that on a 2D polydimethylsiloxane (PDMS) surface. Using confocal laser microscopy and organelle-specific labeling techniques, they could measure the locations of the organelles as the cells migrated either in a 2D platform or in a 3D column. The results of these analyses revealed that the slime mold cells position their centrosome and the nucleus in a non-random orientation as they moved. Generally, the centrosome was positioned towards the rear of the nucleus relative to the direction of movement. By contrast the nucleus relocated itself towards the front of the cell, which was expanding into new territory. The same phenomenon was observed in both the 2D and the 3D environments.
These data demonstrate that the centrosome is indeed orchestrating the microtubules in relation to the position of the nucleus in order to push the cells forward up a concentration gradient. The results should prove useful when extrapolated to studies of cell migration during wound-healing events in humans.