Research Highlights
Enamel Matrix Derivative Enhances the Odontoblastic Differentiation of Dental Pulp Stem Cells via Activating MAPK Signaling Pathways
Institutions:
Chinese Medical University, Liaoning Provincial Key Laboratory of Oral Diseases, Shenyang, China
Team:
Zhang B., Xiao M., Cheng X., Yu B., Chen H., Yu Q., and Qiu, L.
Application:
Creating and injectable hydrogel for the tracking of dental pulp regeneration via stimulation of stem cells
Disease Model:
Dental pulp regeneration
Hydrogel:
VitroGel® RGD
Dental pulp is a tissue that can undergo regeneration under certain circumstances. Understanding how this tissue differentiates from stem cells could lead to advanced therapy for damage that occurs as a consequence of bacterial infections, trauma, or chemical abrasions. We do know that pulp differentiation can be triggered by enamel matrix proteins (EMPs). These are a complex of proteins that are synthesized and secreted by inner enamel epithelial cells from enamel organ and the epithelial root sheath. During the development of the crown of teeth, EMPs are secreted, regulating the mineralization and maturation of enamel. Additionally, Enamel Matrix Derivative (EMD) is another critical signal of cellular differentiation during odontogenesis. This substance is a complex mixture of components, typically obtained by extraction from fetal pig tooth material and used in dentistry to stimulate tissue regrowth surrounding teeth. In this study, researchers sought to determine whether EMD could promote the differentiation of dental pulp stem cells (DPSCs) in vitro and in vivo, and if so, what the underlying molecular mechanisms are. One possibility is that mitogen-activated protein kinase (MAPK) signaling plays a key role in this dental cell differentiation. Thus, another objective of the current work was to utilize both in vitro and in vivo assays to ascertain the exact molecular mechanisms involved in MAPK-directed cellular differentiation in DPSCs.
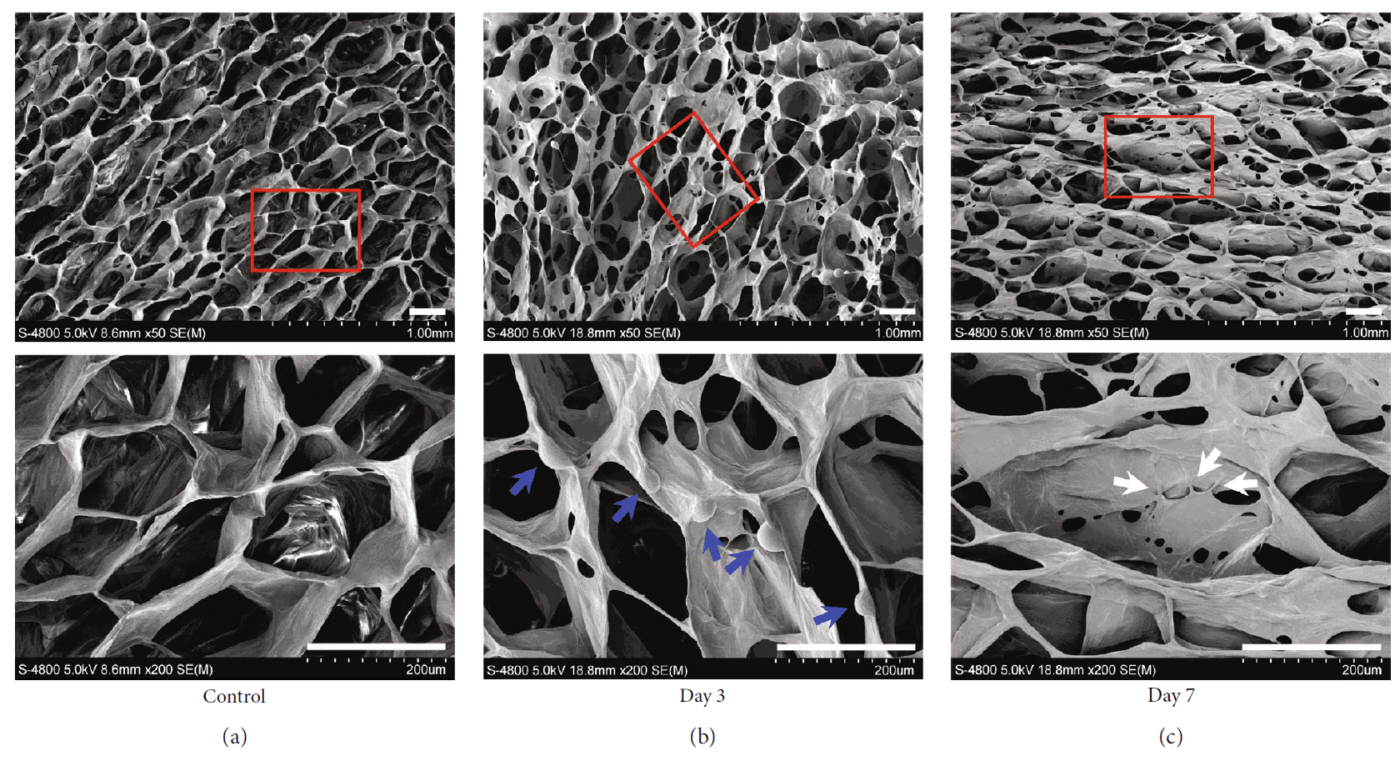
A team of researchers from the Liaoning Provincial Key Laboratory of Oral Diseases and affiliated laboratories explored the influence of EMD on the differentiation of DPSCs in vitro. Here, using RT-PCR, they found that the expression levels of odonto/osteogenic-related marker loci were significantly upregulated by the application of 100 ug/mL of EMD. The team then sought to test whether the effects could also be seen in an in vivo model. For the latter, the team used VitroGel 3D RGD as an injectable scaffold. DPSCs were resuspended at a density of 1 x 106 cells/mL and then gently mixed with a VitroGel 3D RGD:dilution solution:1:3 suspension in a 2:1 volume/volume ratio. This hydrogel preparation was then incubated at room temperature for 20 minutes, and then cultured for 3 and 7 days with or without seeding by EMD. Then the hydrogels were frozen at –80°C and visualized by scanning electron microscopy. They observed the growth of DPSCs cultured in VitroGel 3D RGD, confirming that the Vitrogel 3D RGD had good biocompatibility with DPSCs. Consequently, the cell-hydrogel mixtures were subcutaneously injected into female immunodeficient mice. After six weeks, the effects of EMD on odontoblastic differentiation of DPSCs in this in vivo setting could be analyzed. They found that the EMD group had abundant dentin matrix deposition and more newly formed blood vessels compared to the control group. Lastly, the researchers confirmed that the MAPK signaling pathway could be implicated in the DPSC differentiation through Western blotting assays. In summary, this study concluded that EMD could be a viable alternative to root canal therapy in the treatment of dental pulp degeneration and that a specific kinase signaling pathway was identified as the ultimate molecular mechanism for the EMD-promoted differentiation of DPSCs.
Read the publication:
Related Products: