Research Highlights
Glucose Metabolism in Obese Mice can be Improved by Implantation with Photoactivated Adipose Stem Cells
Institution:
Southwest Medical University, Luzhou, Sichuan, China
Team:
Zhu L., Feng Z., Shu X., Gao Q., Wu J., Du Z., Li R., Wang L., Chen N., Li Y., Luo M., and Wu J.
Application:
In vivo implantation of adipose stem cells to ameliorate glucose intolerance in obese mice
Disease Model:
Type 2 Diabetes
Hydrogel:
VitroGel® RGD
Recent research has implicated enhanced adipose tissue macrophages (ATMs) in the exacerbation of the inflammatory response affiliated with obesity and metabolic dysfunction during the course of Type 2 diabetes. The influence of these cells can be ameliorated by the transplantation of stem cells derived from subcutaneous adipose tissue from healthy individuals. However, therapeutic clinical challenges remain in this regard because of problems such as low cell retention, cell survival following implantation, and insufficient homing of the cells to their target sites. Therefore, improved strategies for implantation protocols are a high priority. Previously, it had been shown that intravenous infusion of mesenchymal stem cells (MSCs) or adipose-derived stem cells (ASCs), coupled with photoactivation at specific visible light wavelengths, could improve the adipose microenvironment with respect to glucose metabolism. In the current study, the team from China’s Southwest Medical University further explore this phenomenon by examining the specific contributions of ASCs and photoactivation following transplantation into visceral epididymal adipose tissue (EAT) in obese mice. In addition, they were able to track the migration routes and lifetimes of transplanted cells using in vivo fluorescent labeling.
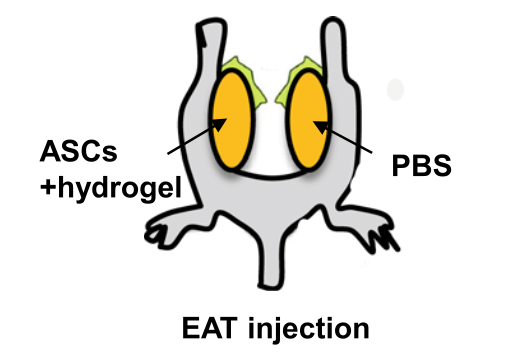
Obesity was induced in male rats by feeding them a high-fat diet for 16 weeks. Mouse ASCs were then harvested and cultured from the subcutaneous fat of healthy rats. These were identified by specific cell-surface markers using FACS. Once cultured, the cells were photoactivated by 30-min illumination regimens at one of three visible-light wavelengths (monochromatic yellow, red, or blue); another set of cells remained untreated as a control. Next, about 2 million such cells were mixed with an 80 µL working solution of VitroGel® RGD hydrogel as a matrix for subcutaneous delivery into the obese subjects. The treatment continued in the obese mice along with their high-fat diets for another 10 weeks. At this time, tissue samples were taken and analyzed for gene expression levels (by qRT-PCR), glucose tolerance (by a glucose tolerance test, or GTT), insulin intolerance (by an ITT), and cell trafficking patterns (by flow cytometry and immunohistochemistry). The results showed three intriguing patterns. First, regardless of whether the treatment stem cells had been photoactivated, obese mice receiving ASCs and hydrogel displayed more rapid glucose disposal during the GTT compared to those who only received hydrogel alone. Second, although the type and degree of photoactivation did not affect the GTT or ITT after 10 weeks of implantation therapy, the light-treated group showed significantly lower blood glucose levels after 4–9 additional weeks post-treatment, demonstrating the prolonged therapeutic effect of the light treatment. And third, migration towards either light-treated or non-light-treated ASCs by inflammatory macrophages was significantly hindered compared with the control group. These findings suggest that improved means to mitigate the metabolic complications associated with Type 2 diabetes may be on the horizon.
Read the publication:
Related Products: