Research Highlights
Mesenchymal Stromal Cell-Derived CXCL12 Affects Tumor Growth by Macrophage M1 to M2 Polarization
Institution:
Department of Clinical Pathology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
Team:
Babazadeh S., Nassiri S. M., Siavashi V., Sahlabadi M., Hajinasrollah M., and Zamani-Ahmadmahmudi Z.
Application:
Identification of a new role for MSC-derived CXCL12 in M2 phenotypic switching of macrophages, affecting tumorigenesis in breast cancer cells
Disease Model:
Breast Cancer
Hydrogel:
VitroGel® RGD
Studies over the past two decades have made it clear that macrophages display great functional and phenotypic diversity. This diversity widely depends on their pro-inflammatory M1 or anti-inflammatory/tissue-remodeling alternatively activated M2 polarization, a status which macrophages can oscillate between that results in a broad range of functions, including several chronic pathologies within tumor cells. Given recent evidence indicating macrophages are a primary component of infiltrated leukocytes in malignant tumors and that presence of M2 macrophages correlates with the worsening of certain neoplastic conditions, recognizing what the polarization status of macrophages is an important factor for determining disease status. Another important factor commonly localized to injury sites and tumors are mesenchymal stromal cells (MSCs), which can promote tumorigenesis, chemoresistance, and tumor growth. In this manuscript, Babazadeh and colleagues have described that MSC-released C-X-C motif chemokine ligand 12 (CXCL12), also known as stromal cell-derived factor 1 (SDF-1), is inducible in certain pathological states including tumor formation. Moreover, induction of CXCL12 results in a variety of biological functions including proliferation, cell migration, differentiation, or apoptosis.
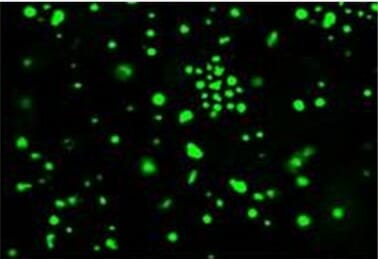
To illustrate that MSC-derived CXCL12 dictates macrophage polarization status, the investigators developed an MSC cell line with or without CXCL12 expression (MSCCXCL12+/+, MSCsCXCL12-/-). Once the MSC cells were constructed, they were subsequently co-cultured with macrophages harvested from bone marrow on the VitroGel Hydrogel matrix, which precisely mimics the natural extracellular matrix (ECM). To achieve this co-culture system, macrophages were stained, mixed with a 4T1 (breast cancer cells) cell mixture, and then combined with a VitroGel solution that was then plated in 96-well plates. Using the VitroGel Hydrogel co-culture system, multicellular tumor spheroid formations can be observed as an indication of tumor growth or suppression. Analysis of this co-culture system revealed that M2 macrophage markers (IL10, IL4, TGF-β, IL6) in BMDM cells were dramatically reduced in cells without CXCL12, and M1 macrophages were markedly induced, indicating CXCL12 may mediate macrophage polarization. To assess the impact of this phenotypic switching on tumor induction in vivo, a mixture of 4T1 plus BMDMs co-cultured with MSCsCXCL12+/+ or MSCCXCL12-/- were injected into the mammary fat pads of mice. Analysis of these mice illustrated that co-injection of MSCsCXCL12+/+-primed macrophages substantially increased tumor induction potency by 4T1 cells compared to MSCCXCL12-/- co-injection, and these results were consistent with observations of greater multicellular tumor spheroid formations as well. These findings indicate a new role for MSC-derived CXCL12 in the ability to promote macrophage polarization to affect their function in tumorigenesis.
Read the publication:
Related Products: