Research Highlights
The Good Side of Bad Cholesterol
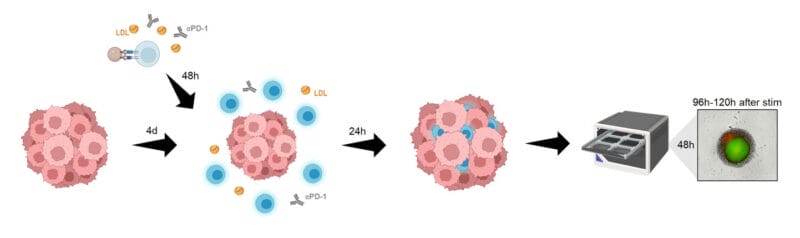
Institutions:
University Hospital Regensburg, Germany
Team:
Babl, N., Hofbauer, J., Matos, C., Voll, F., Menevse, A.N., Rechenmacher, M., Mair, R., Beckhove, P., Herr, W., Siska, P.J., Renner, K., Kreutz, M., & Schnell, A.
Disease Model:
Colorectal Cancer
Biomarker:
Cyto3D® Live-Dead Assay Kit
Cholesterol, in the form of LDL, demonstrates an intriguing ability to stimulate the immunotherapeutic potential of T-cells against tumors.
Immunotherapy has been heralded as a significant step forward in our ability to combat cancer. The notion is that we can use the body’s own immune system, and/or key pieces of information regarding how cancer cells are perceived by the immune surveillance process, to target and incapacitate budding tumors. One strategy that has shown promise in some, but not all patients, is to target the PD-1 (programmed cell death 1) receptor. This protein normally keeps T-cells from attacking other cells in one’s own body. But its ligand PD-L1 appears to be highly upregulated in some cancer cells, making them invisible to T-cell attack. Certain cancer drugs such as nivolumab (Optivo) work by blocking the PD-1/PD-L1 interaction, thereby stimulating the natural tendency of the immune system to kill aberrant cells, such as those in a tumor.
Some substances have been shown to be biomarkers, or “broadcasters” of this inhibitory interaction that prevents maximal efficacy of immunotherapy. The simple molecule cholesterol turns out to be one of these biomarkers. Adding cholesterol, in some studies, can improve the outcome of patients undergoing immunotherapy for cancer. But the reason for this is unclear, and clearly, too much cholesterol is bad for the body. Thus, a team of scientists from Germany sought to clarify the complex interaction between cholesterol and T-cells, with the hope that this understanding could improve our exploitation of immune therapy in the field of oncology.
The authors performed a series of experiments in which serum levels of LDL (low-density lipoprotein), or “bad” cholesterol was correlated with T-cell phenotype and metabolism. They used CD-4+ and CD-8+ T-cells and cultured them in vitro. They added LDL and watched how well the T-cells responded, including in the context of being co-cultured with tumor spheroids (3D cell models that mimic the characteristics of cell aggregates). Using flow cytometry, ELISA, and oxygen and pH monitoring, they could track how well the T-cells––and the spheroids––were responding to various levels of LDL supplementation. The viability of the spheroids was assayed using TheWell Bioscience’s Cyto3D® Live-Dead Assay Kit.
With these techniques, the authors found that high amounts of LDL caused the downregulation of PD-1 in the T-cells, and that these T-cells displayed a more “normal” phenotype, with a decrease in the production of reactive oxygen species (ROS). These T-cells, when incubated with spheroids that were mimics of colorectal cancer (CRC) cells, could indeed inhibit their viability. The authors suggest that this increased anti-cancer effect, stimulated through higher levels of cholesterol, could be mediated by T-cells that were less “exhausted” and more able to carry out their tumor cell killing function. However, before specific therapeutic recommendations are made regarding cholesterol, more studies will be needed to fine tune our appreciation of how this complex cellular and molecular dance is carried out.


