Research Highlights
Tracking Down the Culprit Responsible For AML
Institutions:
Jilin University, China
Team:
Liu, M., Yu, B., Tian, Y., and Li, F.
Disease Model:
Acute Myeloid Leukemia
Hydrogel:
VitroGel® RGD
A combination of a gene expression network analysis and in vitro assays implicates regulation and modification of a particular enzyme, WTAP, as a source of tumorigenesis in acute myeloid leukemia (AML).
One of the several forms of malignant tumors that has an unknown pathogenesis is acute myeloid leukemia (AML), which is nonetheless the products of accumulated genetic mutations. A suspected route through which such mutations can occur is through RNA alterations, such as N6-methyladenosine (m6A) methylation. Aberrant control of this process is known to trigger tumorigenesis in a variety of cancers, particularly with relation to long non-coding RNAs (lncRNAs). Prior to this paper, the exact relationship between RNA modification dysregulation and AML was not clear.
A team of cancer biologists from Jilan University examined this connection, focusing on the m6A methyltransferase enzyme WTAP. These authors first examined RNA expression profiles from international databases. This established a background interaction network of lncRNAs, miRNAs (microRNAs), and general mRNAs. Then the authors examined specific RNA expression levels in actual cells, comparing normal human bone marrow stromal cells (cell line HS-5) and the AML cell lines THP-1 and HL-60. The goal was to determine the differential expressions of these three classes of RNAs and determine relationships that were critical in AML pathogenesis.
Using targeted RNA amplification via RT-PCR, the authors screened out key genetic loci that exhibited strong differential expression patterns between normal and AML cells. One particular interaction subnetwork stood out, the SUCLG2-AS1-hsa-miR-17-5p-JAK1 axis. Importantly, SUCLG2-AS1, a lncRNA that had been previously demonstrated to play a key role in kidney cancer, was a main component of this axis. The authors’ RNA expression data showed that SUCLG2-AS1 and JAK1 (Janus kinase, a tyrosine kinase that is generally found in association with cytokine receptors) were both downregulated in AML cells compared with normal cells, while other small RNAs were upregulated in AML cells compared with normal cells.
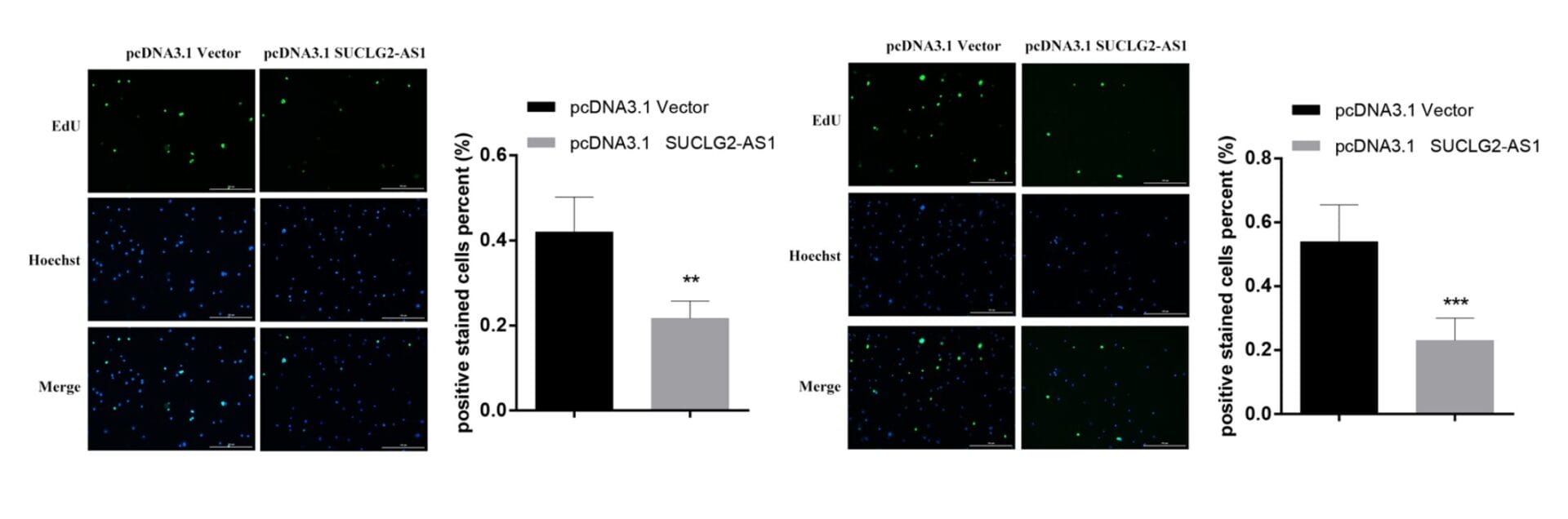
The authors then performed functional assays on live cell lines. They transfected the AML cell lines with a vector that would cause an upregulation of SUCLG2-AS1 in these cells. Using transwell assays in which the cells were embedded in TheWell Bioscience’s VitroGel® RGD hydrogel matrix, the team was able to show that the proliferation, migration, and invasion of AML cells overexpressing this locus was significantly decreased. Consequently, these data suggested strongly that this lncRNA inhibits cell invasion and metastasis and induces the apoptosis of AML cells. Further bioinformatic analyses concluded that this RNA acted as a “sponge” for binding to other miRNAs; when this phenomenon was not available then these bindings turn their sights on WTAP, leading to cellular events that promote tumor formation.
The results from this paper reveal a complex network of genetic loci and post-transcriptional modification events that can determine the cellular fate of bone marrow cells, and in particular those that can trigger leukemia. These data also provide a new and valuable therapeutic target, namely WTAP, for the treatment of AML.